A “knockout mouse” is a strain of mice in which a section of their DNA has been deleted. Therefore, they do not have a particular gene. Sometimes, this has disastrous consequences, certain genes they cannot live without and thus they are never born. But when a knockout mouse lives, it can teach us interesting lessons about the function of the gene they are missing.
NOTE: This post and the next have some confusing terminology. SCD1 is the name of a GENE – a stretch of DNA that encodes a protein. Steroyl-CoA desaturase is the name of the enzyme – the gene contains the coding of a protein, in this case an enzyme. I will use SCD1 and Steroyl-CoA desaturase somewhat interchangeably as does the scientific literature.
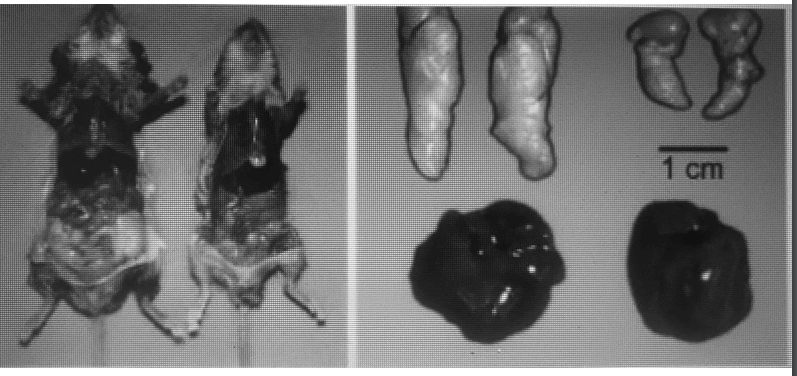
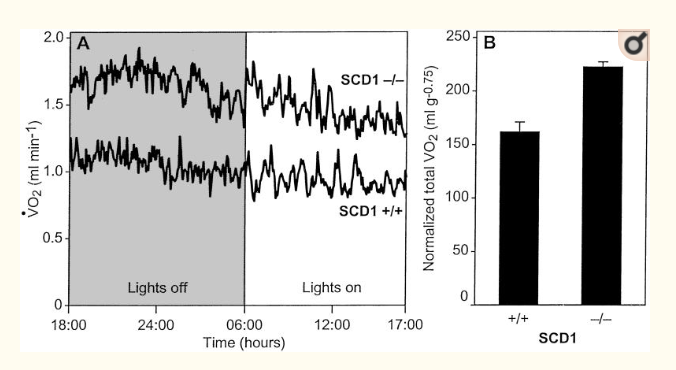
There is a gene in mice called SCD1 which produces an enzyme called Stearoyl-CoA desaturase. The enzyme has the single function of turning a long chain saturated fat (such as stearic acid found in beef fat) into a monounsaturated fat (similar to the oleic acid found in olive oil). In this paper, from 2002, it was shown that mice who cannot convert saturated fat into unsaturated fat have a high metabolic rate and remain lean.
The full paper.
There are only two sources of fat in the body – fat obtained from the diet or fat produced in the body by a process called de novo lipogenesis. The end produced of de novo lipogenesis is long chain saturated fat. Once the long chain saturated fat is produced, Stearoyl-CoA desaturase can turn it into monounsaturated fat. The level of the enzyme in fat cells is a major regulator of the proportion of saturated fat to monounsaturated fat that is stored in our fat cells. In turn, the ratio of saturated to mono-unsaturated fat controls overall body fatness, as exhibited by these mice.
The regulation of Stearoyl-CoA desaturase is very interesting. First and foremost, we produce more of it in response to dietary saturated fat and less of it in response to unsaturated dietary fat. This is presumably the body’s way of trying to achieve balance. If you are eating a lot of highly saturated fat, you will convert a lot of it to unsaturated fat for storage. Conversely, if you eat a lot of unsaturated olive oil, enzyme levels will drop and most of the fat you create during de novo lipogenesis will be saturated. In healthy adults, it seems like the trend is towards a very roughly 50:50 mix of long chain saturated and unsaturated fats in our fat stores along with a proportion of polyunsaturated fats.
This fat blend should produce a reasonable amount of superoxide at the bottleneck in the the mitochondrial electron transport chain to produce physiological insulin resistance as a signal to the body that stored fat is being burned. This is the balance that the enzyme is trying to reach. ROS is the signal. If the stored fat is too unsaturated, physiological insulin resistance will not be achieved and the tendency will be for the fat cells to continue to listen to insulin and continue storing fat even though fat is plentiful. If the stored fat is too saturated, physiological insulin resistance will be achieved quickly and regularly, insulin will be ignored by the fat cells, fat burning will continue and the animal will become very lean. This is exactly what happens when Stearoyl-CoA desaturase is deleted from the mouse genome.
The other interesting thing about the regulation of Stearoyl-CoA desaturase is that it is upregulated in response to dietary sugar. Humans evolved as hunter-gatherers. Finding a large crop of ripe fruit would have been a time to feast and store calories. When the sugar is ingested, Stearoyl-CoA desaturase levels would increase, the fat produced by de novo lipogenesis from the fruit would be more unsaturated than not, physiological insulin resistance would be avoided, fat would be stored. This is a likely mechanism for how a sugary diet causes obesity.
