NADH and NADPH are electron carriers. They are the “wires” that conduct electrical power through your cells. When they have the electrons they are the H version: NADH or NADPH. When the give off the electrons, they become NAD and NADP. They are interconvertible. NAD kinase converts NAD to NADP by adding a phosphate group and a phosphatase reverses this.
The very first thing that we could call a living cell had two alternative pathways for how we deal with glucose. One is called glycolysis and it produces NADH as a side-effect. The other is called the pentose phosphate pathway (PPP) and it produces NADPH as a side effect. These pathways are the most ancient metabolic pathways. The first ones.
This is a switch. If the cell needs to be anabolic – ie activated immune cells or adipose cells in obesity, it switches the PPP ON and creates NADPH. One of the main regulators of PPP is Nrf2, the master controller of antioxidant defense. Nrf2 turns on the enzymes that produce glutathione as well as the antioxidant enzyme glutathione peroxidase. With those things in place plus a steady supply of NADPH from PPP you can steadily remove ROS.

In eukaryotes (things with a mitochondria, MUCH later on) the NADH came to be shuttled into the mitochondria to produce ATP. It has no other functional role in the cytoplasm, where it is produced. The NADPH, however, fuels several cytoplasmic processes:
- It is the fuel that drives antioxidant enzymes. Glutathione peroxidase is the first line of defense against free radicals and converts NADPH back to NADP for each one neutralized.
- It is the fuel to generate ROS. NADPH powers the enzymes NOX2 and NOX4, called NADPH Oxidases, who are the SOURCE of pro-inflammatory free radicals. Biology is tricky like that.
- It is the fuel for growth. Fatty Acid Synthase drives the process of De Novo Lipogensis (making new fat). It runs off of the power of NADPH. Nucleic acid synthesis also requires NADPH as does glutamate-dehydrogenase, an anabolic enzyme. Growthy.
So the same fuel source in the same part of the cell drives three interrelated processes: growth, inflammation and anti-oxidant defense. All of this is driven by the PPP. In How We Handle Hypoxia I wrote, “But only Nrf2 – the good guy – is implicated in increasing expression of G6PD, the controlling enzyme of the PPP. G6PD was already known to be a diet-inducible lipogenic enzyme back in 1959.” Nrf2 is also strongly implicated in cancer progression. (Roja De La Vega, 2018) Growthy. Because antioxidant defense and making fat use the same fuel.
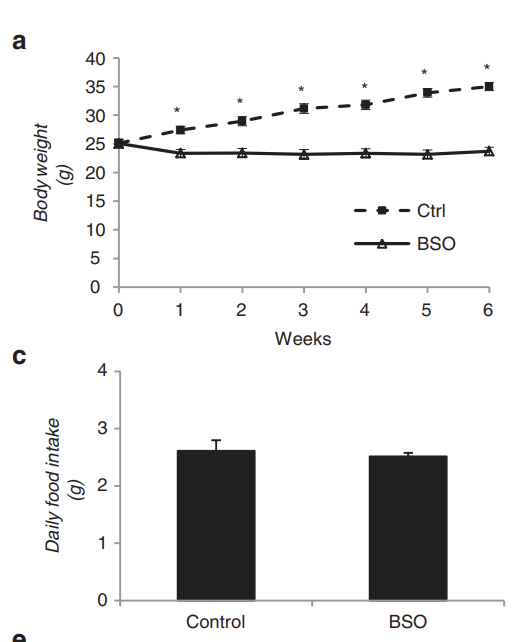
This helps clear up some confusing scientific results. Mice who lack Nrf2 are protected from insulin resistance. If you use the compound BSO to block glutathione synthesis you prevent all aspects of the metabolic syndrome in diet-induced obesity. Same calories, less fatter.
Glutathione is a circulating redox buffer which can “recharge” NADP back to NADPH to fuel antioxidant defense or growth, depending. Glutathione can be but is not necessarily use in antioxidant defense by glutathione peroxidase. Cancer cells are full of glutathione. Growthy.
Here’s a fun diagram of how when NADPH is oxidized to NADP, the glutathione gets reduced. This can actually go both ways. When NADPH levels start to drop, it is replenished by electrons from reduced glutathione.
The antioxidant vitamins C and E can also replenish NADPH. Chronic supplementation of vitamin C or E can be used to create insulin resistance in rats.
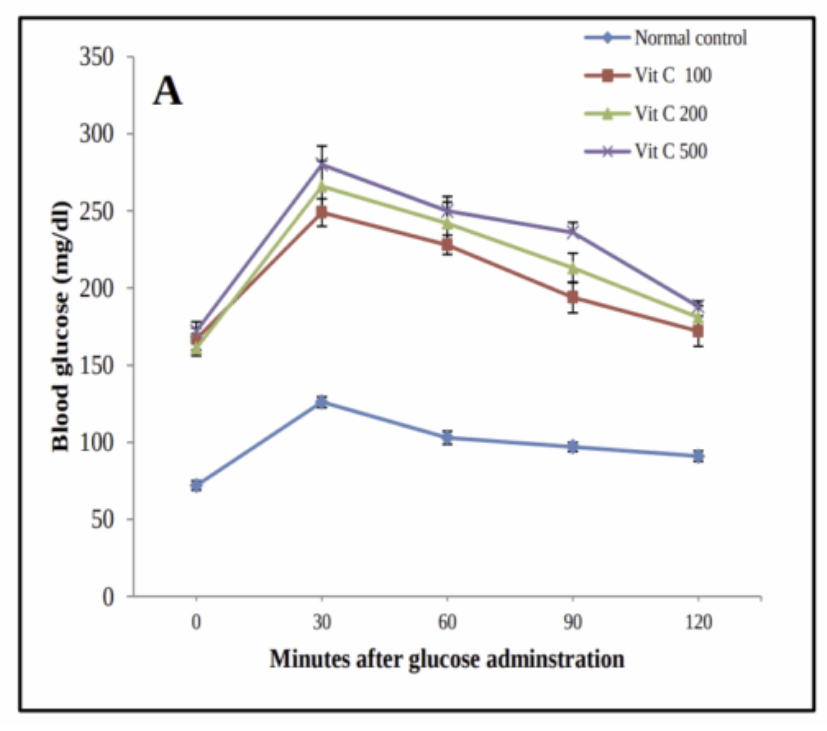
This paper is really neat because it shows so logically how all of this works. The antioxidants are constantly bombarding the NADPH redox buffer with electrons. This causes redox imbalance, activating Nrf2.
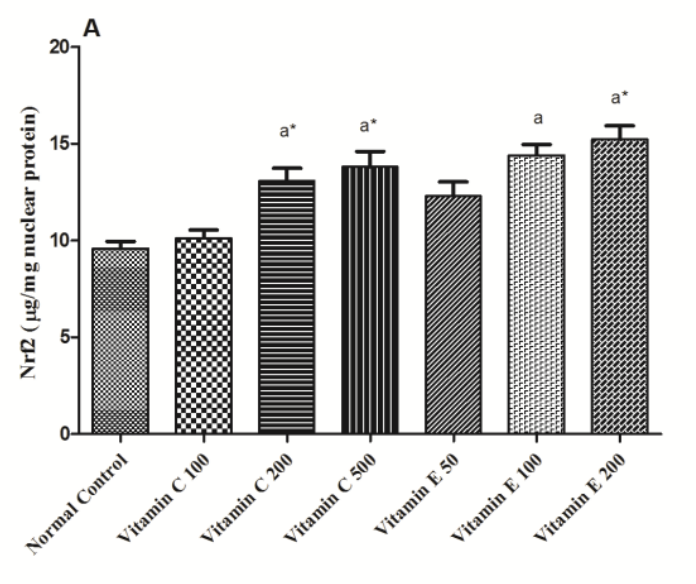
The NADPH buffer isn’t large enough to hold all of the electrons, so Nrf2 increases production of glutathione, the body’s master backup-NADPH-warehouse.
(GSH is shorthand for reduced glutathione. tGSH means reduced plus oxidized glutathione.)
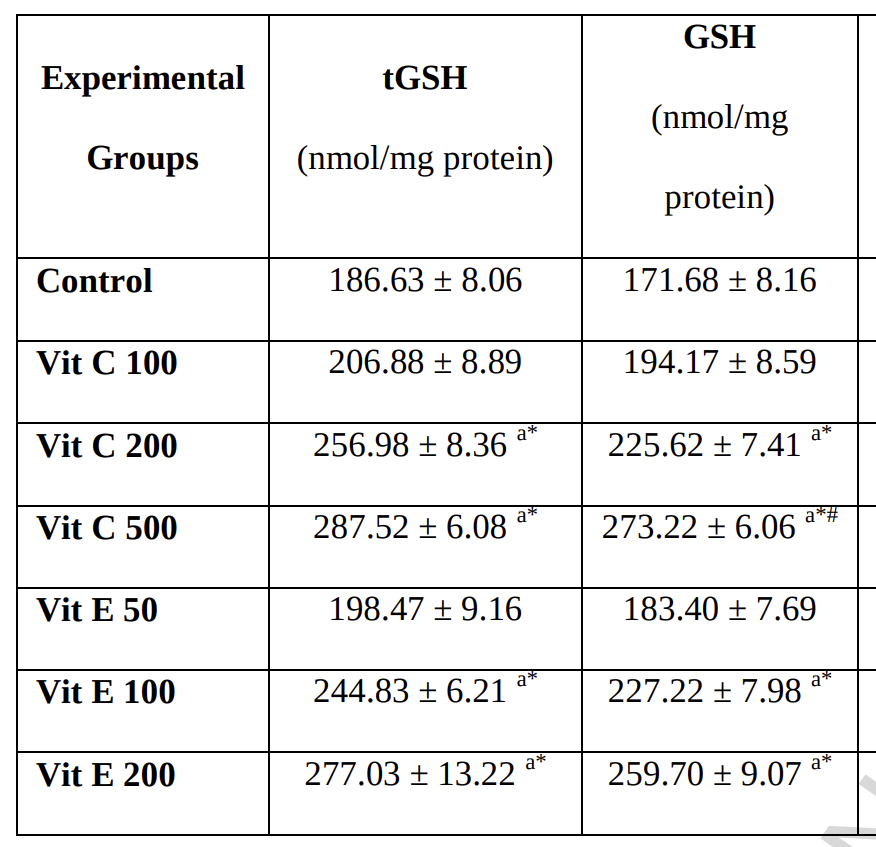
So with a whole warehouse of NADPH replenishing glutathione, NADPH reserves are perpetually high and the cell are growthy: insulin resistant. Insulin should cause us to burn glucose oxidatively, but that fails when cells are switched into growth mode.
PUFA Oxidize
Of course this is relevant to PUFA metabolism because polyunsaturated fats are one of the first things to auto-oxidize in the case of free radical induced oxidative stress. But do the lipid peroxides per se cause metabolic syndrome? These studies suggest that they do not.
The vitamin E fed rats with a fully charged antioxidant defense system are the insulin resistant ones. BSO definitely causes large rises in 4-HNE (Yan, 2006), but the BSO treated mice are the ones who are resistant to diet-induced obesity.
So then why should we avoid PUFA? Read on.
Ali, Mennatallah A., Rasha A. El-Tahan, et al. “The Diabetogenic Effects of Chronic Supplementation of Vitamin C or E in Rats: Interplay between Liver and Adipose Tissues Transcriptional Machinery of Lipid Metabolism.” Life Sciences, vol. 306, 2022, p. 120812, https://doi.org/10.1016/j.lfs.2022.120812.
Ali, Mennatallah A., Rania M. H. M. Eid, et al. “Vitamin C and E Chronic Supplementation Differentially Affect Hepatic Insulin Signaling in Rats.” Life Sciences, vol. 194, 2018, pp. 196–204, https://doi.org/10.1016/j.lfs.2017.12.039.
Findeisen, Hannes M., et al. “Glutathione Depletion Prevents Diet‐Induced Obesity and Enhances Insulin Sensitivity.” Obesity, vol. 19, no. 12, 2011, pp. 2429–32, https://doi.org/10.1038/oby.2011.298.
Kalinina, Elena. “Glutathione-Dependent Pathways in Cancer Cells.” International Journal of Molecular Sciences, vol. 25, no. 15, Aug. 2024, p. 8423, https://doi.org/10.3390/ijms25158423.
Rojo De La Vega, Montserrat, et al. “NRF2 and the Hallmarks of Cancer.” Cancer Cell, vol. 34, no. 1, 2018, pp. 21–43, https://doi.org/10.1016/j.ccell.2018.03.022.
Yan, Jin, and Barbara F. Hales. “Depletion of Glutathione Induces 4-Hydroxynonenal Protein Adducts and Hydroxyurea Teratogenicity in the Organogenesis Stage Mouse Embryo.” The Journal of Pharmacology and Experimental Therapeutics, vol. 319, no. 2, 2006, pp. 613–21, https://doi.org/10.1124/jpet.106.109850.
Zhang, Yu-Kun Jennifer, et al. “Nrf2 Deficiency Improves Glucose Tolerance in Mice Fed a High-Fat Diet.” Toxicology and Applied Pharmacology, vol. 264, no. 3, 2012, pp. 305–14, https://doi.org/10.1016/j.taap.2012.09.014.
