The fatty acid composition of the phospholipids from blood cell membranes have been reported many times in the literature. It is an easy thing to collect and quantify. We think of cell components as a sort of constant. In fact they are very dynamic.
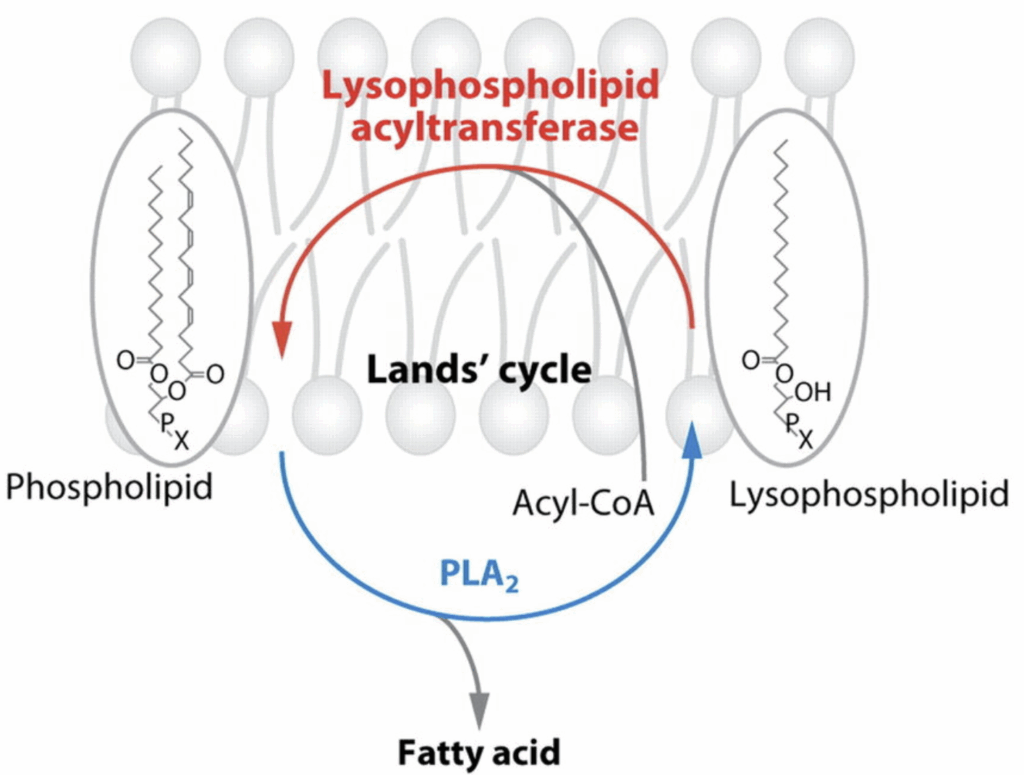
A process called The Lands Cycle is constantly rearranging phospholipids in cell membranes. The phospholipid in the SN-1 position anchors the phospholipid in place. This fat is typically saturated. A phospholipid in the SN-2 position – typically polyunsaturated – is constantly being released by a phospholipase (PLA2) and replaced by an acyltransferase (LPCAT3).
The Half-life Of Membrane PUFA Is Days
One of the most commonly found phospholipids is 16:0, 18:2 PC. This is phosphatidyl choline with palmitic acid in the SN-1 position and linoleic acid in the SN-2 position. Based on studies in rabbits using radioactive tracers (the gold standard), the linoleic acid at SN-2 is released every 1.5 days. The Lands Cycle is a continous, ongoing process.
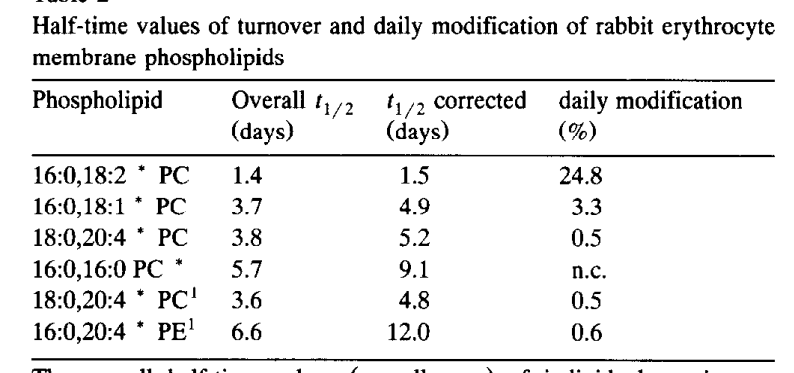
LPCAT3 replaces the fatty acid from the SN-2 position. It uses free fatty acids from your bloodstream, which are largely reflective of your adipose tissue composition in the fasted state but which are affected by fat from a meal in the post-prandial state.
LPCAT3 has a strong preference for linoleic acid. That’s not an accident, that is how the system is designed. Let’s think about WHY.
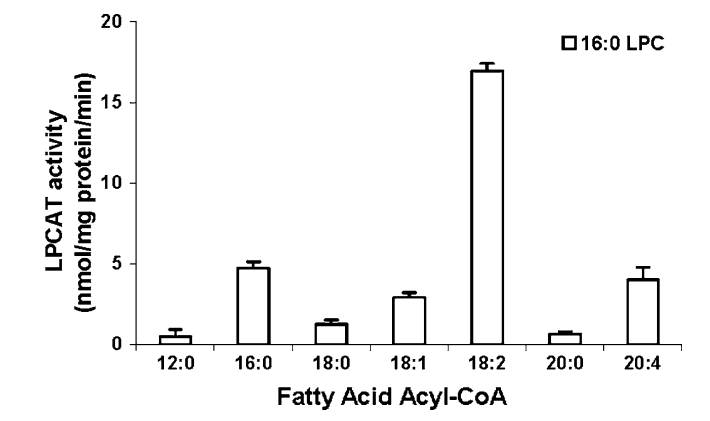
Reducing LA With Carbs
On the eve of my writing this article, exfatloss posted this enlightening chart.
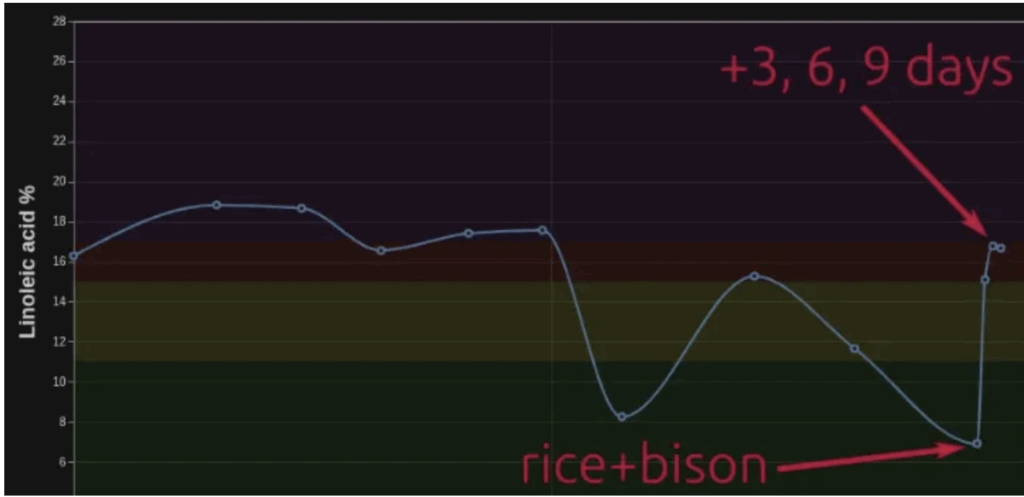
He is using the OmegaQuant complete test every 3 days to measure the fatty acids in his red blood cell phospholipids (RBCP). What he has realized is that when he eats a swampy meal (rice+bison), the level of linoleic acid in his RBCP plunges. When he reverts to a very high-fat, low-protein diet (heavy cream), the levels of linoleic acid revert to baseline. Why?
RBCs are an interesting model. They lack mitochondria and they lack insulin receptors. They don’t do pinocytosis or secrete hormones – both processes that lead to extensive membrane remodeling. Their overall membrane structure is essentially static. These facts eliminate a HUGE number of variables of what could be happening.
Here’s my guess. In human obesity, liver and adipose tend to be glycolytic. This causes lactate export and a steady rise in the lactate/pyruvate ratio over time. Eating the swampy meal, exfatloss is providing the glucose for conversion to lactate. When he removes it, he is removing the substrate for lactate production.
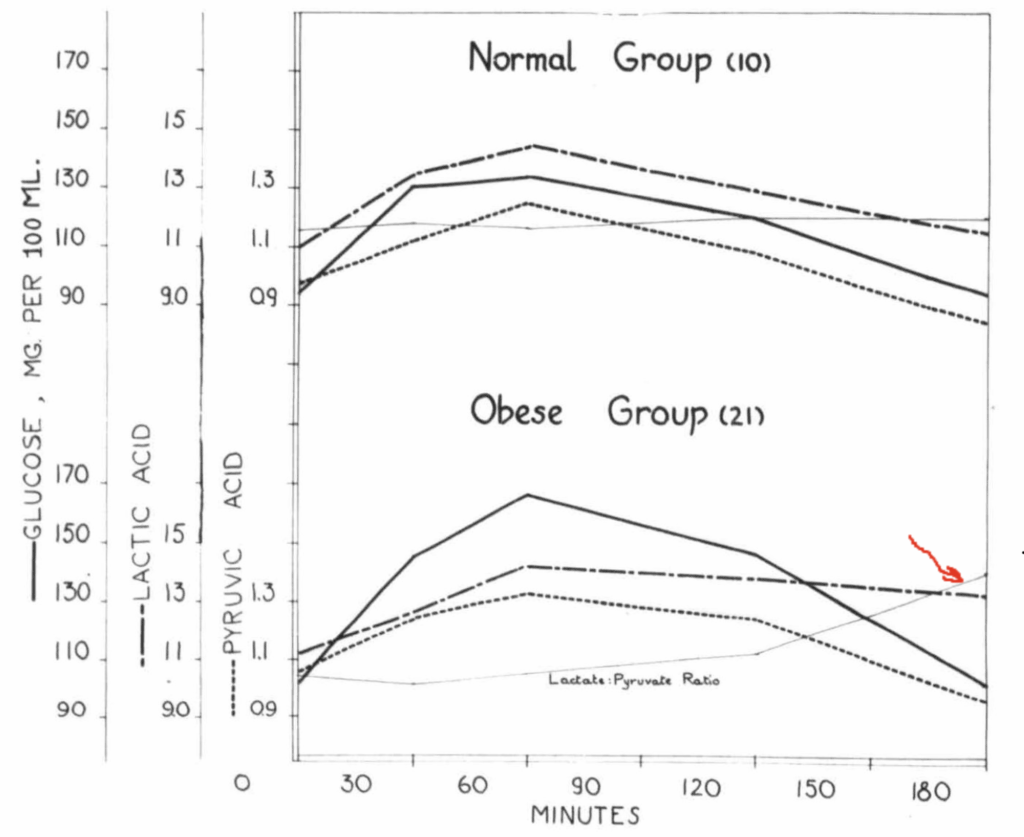
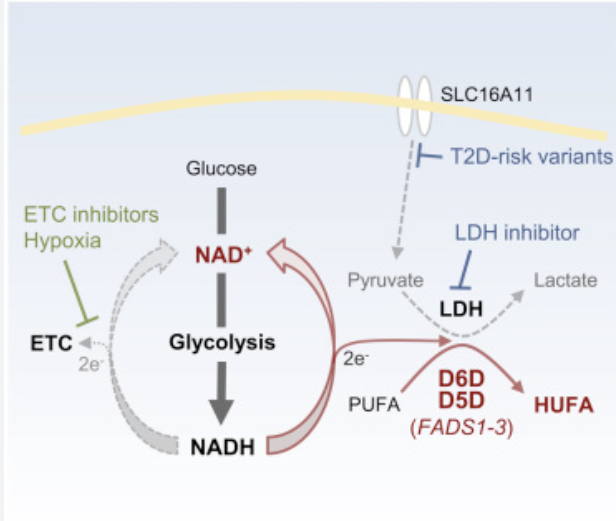
RBCs are entirely reliant on glycolysis to generate ATP, which they need to drive the ion pumps that help them maintain membrane integrity. Glycolysis requires NAD, the oxidized form of NADH. Two NAD are converted to NADH for each molecule of glucose that is run through glycolysis. The RBC then would use the two molecules of pyruvate generated by glycolysis as the electron acceptor, turning them to lactate for export. This regenerates NAD and allows ongoing glycolysis.
In a high lactate/pyruvate state, the RBCs have a problem. Like nearly all tissues, the RBCs import lactate and/or pyruvate and the enzyme lactate dehydrogenase equilibrates the intracellular NAD/NADH ratio based on that. In a world of rising lactate/pyruvate, the RBCs need to find an alternate electron acceptor.
The solution is to release the linoleic acid from the membranes and ferment it using D6D and D5D. These enzymes add unsaturated bonds to the oleic acid. After both of them have done their thing, two molecules of NAD are regenerated from NADH per molecule of linoleic acid. The linoleic acid is now arachidonic acid, which can diffuse out of the cell or be converted to an eicosanoid and released by a transporter protein. This cycle is a continuous ongoing cycle of importing linoleic acid; using it as the terminal electron acceptor; and release.
When exfatloss switched back to a cream based diet, his lactate/pyruvate ratio was no longer affected by the meal. LPCAT3 replaced the linoleic acid over the next three days. Homeostasis (in this context) was restored.
Avoid it.
Arendt, E. C., and C. J. Pattee. “STUDIES ON OBESITY II. BLOOD PYRUVATE AND LACTATE CURVES IN OBESE AND NORMAL SUBJECTS AFTER INGESTION OF GLUCOSE.” The Journal of Clinical Endocrinology & Metabolism, vol. 16, no. 3, 1956, pp. 375–79, https://doi.org/10.1210/jcem-16-3-375.
Bankaitis, Vytas A. “The Cirque Du Soleil of Golgi Membrane Dynamics.” Journal of Cell Biology, vol. 186, no. 2, July 2009, pp. 169–71, https://doi.org/10.1083/jcb.200907008.
Kazachkov, Michael, et al. “Substrate Preferences of a Lysophosphatidylcholine Acyltransferase Highlight Its Role in Phospholipid Remodeling.” Lipids, vol. 43, no. 10, 2008, pp. 895–902, https://doi.org/10.1007/s11745-008-3233-y.
Kim, Wondong, et al. “Polyunsaturated Fatty Acid Desaturation Is a Mechanism for Glycolytic NAD+ Recycling.” Cell Metabolism, vol. 29, no. 4, 2019, pp. 856-870.e7, https://doi.org/10.1016/j.cmet.2018.12.023.
Van Den Boom, M. A. P., et al. “In Vivo Turnover of Phospholipids in Rabbit Erythrocytes.” Biochimica et Biophysica Acta (BBA) – Lipids and Lipid Metabolism, vol. 1215, no. 3, 1994, pp. 314–20, https://doi.org/10.1016/0005-2760(94)90059-0.

Mild correction: rice+bison was not a swampy diet, I technically called it “ex150hclflp.” I chose the bison because ~150g of bison will have 5-7g of fat, with the amount from the rice added, maybe another few grams. But I suspect that I was <10g fat total for most of the days.
That said, it doesn't seem to make a difference for your hypothesis here, any high-glucose diet would've provided the fuel to make lactate?
I think so. I just meant swampy as in combining starch and protein.
Doesn’t this mean we should want to consume linoleic and alpha-linolenic acid, since they’re a sink for backed up electrons, effectively reducing the NAD+/NADH ratio? I see why this means we should avoid the D6D and the D5D HUFA products, but this cuts the opposite way for the input PUFAs.
https://fireinabottle.net/linoleic-acid-is-stealing-your-oxygen/