Here’s the big theory. Desaturase and cytochrome P450 enzymes in the ER compete for oxygen with the mitochondria. This is part of a switch to glycolysis that recycles the cytoplasmic NADH back to the NAD+ that glycolysis requires. If you’re going to do glycolysis, there is no need to allow oxygen into the mitochondria.
As I’ve said, the adipose cells of obese adults are glycolytic. (Jansson, 1994) Glycolysis is fundamentally anabolic and makes things growthy. The “state” of the adipose cell is one that favors retaining carbon rather than burning it. One of the levers an adipose cell can use to grow is to restrict oxygen from reaching the mitochondria. If you want the fuel to build up you have to close the damper.
In mammals, oxygen use in the ER is the damper.
The theory is speculative but has a lot of evidence supporting it. I’d like to prove it.
- PUFA desaturation allows ongoing glycolysis. (Kim, 2019)
- Activity of desaturase enzymes predict progression to diabetes in human adults; mendalian randomization suggests causality. (Janine, 2011) I haven’t heard a competing hypothesis as to why.
- Blocking desaturase enzymes in cancer cells causes a massive drop in both their oxygen consumption and rate of glycolysis. (Hasegawa, 2024; Luis, 2021; Zhou, 2021)
- Linoleic acid availability causes cells to increase oxygen consumption WHILE becoming glycolytic. (Arslan, 1984)
- Adipose tissue of obese humans has elevated desaturase activity. (Warensjo, 2009)
- Adipose tissue of obese humans is glycolytic. (Jansson, 1994)
- Reduced activity of any of SCD1, SCD2, D6D, D5D reduces insulin resistance and/or obesity in rodent models. (Ntambi, 2002; Hucik, 2019; Yashiro, 2016; O.Neill, 2020)
You were probably shown this lie in high school biology. Probably again if you took bio 101. In this image the various organelles all get their own space. The mitochondria are fat, sausage shaped organelles floating free from the Endoplasmic Reticulum (ER).
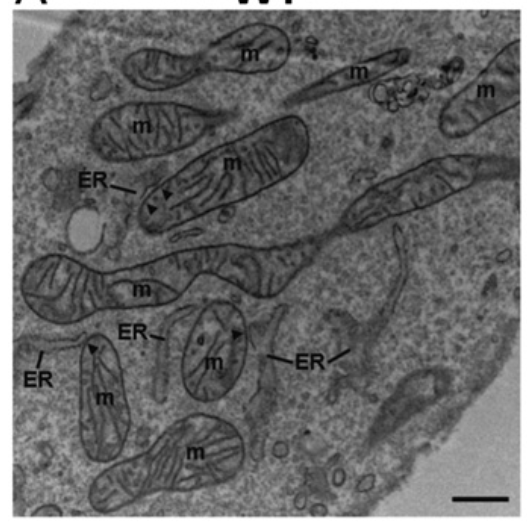
The REALITY is a bit different. The networks of ER and mitochondria are instead INTERTWINED. Here is a cross section of a cell taken with an electron microscope. You can see that the ER and the mitochondria (m) swim in the same waters.
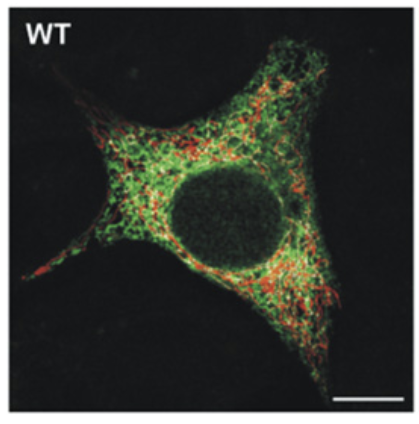
We can zoom out and see this on a whole cell levels using confocal fluorescence microscopy. The red are mitochondria and the green is ER.
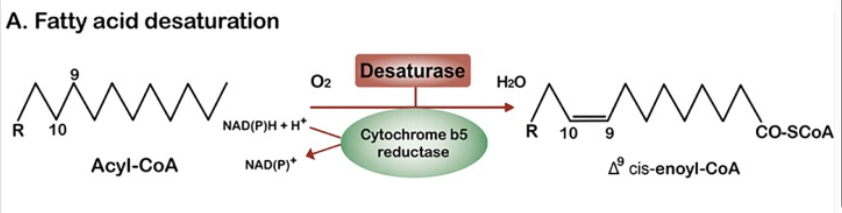
This is relevant because the mitochondria and the ER are the main consumers of oxygen within the cell. No one ever told you that the ER uses oxygen? The ER hosts a suite of oxygen consuming enzymes, including the fatty acid desaturases SCD1, SCD2, D6D and D5D; plus cytochrome P450s such as CYP4a11. Here is the general equation for how fatty acid desaturation works. A double bond is added to a lipid and in the process a molecule of NADH is converted to NAD+ and a molecule of O2 is converted to H2O.
As fatty acids (as acyl-CoAs) diffuse through the cytoplasm they have three possible fates (oversimplified, but…):
- Diffuse to the mitochondria where CPT1 activates them for mitochondrial oxidation by making them an acyl-carnitine.
- Same as 1, except on the way to the mitochondria, they get desaturated in the ER. This would be very common for stearic acid. SCD1 in the ER converts it to oleic acid as it diffuses in, directly competing for the oxygen that the mitochondria needs to burn the oleic acid.
- It gets converted to an oxylipin and exported from the cell for elimination in the urine. This is an oxidative fermentation.
In fermentation, electrons from NADH are transferred to an organic acceptor molecule, regenerating NAD⁺ without involvement of O₂ or the electron-transport chain.
Berg, Tymoczko & Stryer, Biochemistry (9th ed.)
Let’s dig into number three a little more. The pathway is below. Liver cells can take in linoleic acid via the fatty acid transporter CD36. D6D and D5D add double bonds to convert it to Arachidonic Acid (AA). Each desaturation step consumes an oxygen. CYP4A11 also consumes oxygen to convert the AA to 20-HETE. That is 3 molecules of oxygen consumed in the fermentation. The 20-HETE can then be glucuronidated to 20-HETE glucuronide and exported from the cell by MRP2.
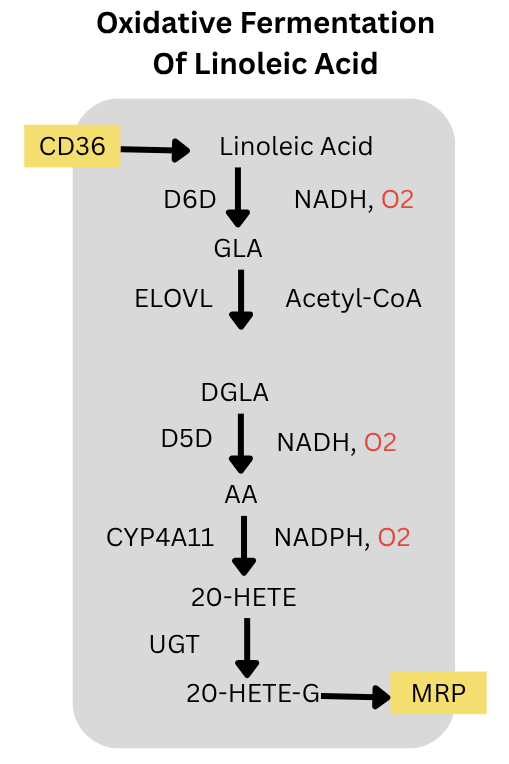
Since the PUFA is fermented and exported, the rate of this process is not limited by the mitochondria’s ability to oxidize the incoming fatty acid. The process can go as fast as the enzymes allow it to go given the quantify of linoleic acid in the free fatty acid supply. This is ongoing.
The enzymes at every link of this pathway; CD36, D6D, ELOVL5, D5D, CYP4A11, UGT enzymes and MRP2; are controlled by PPARa. (Barbier, 2003; Goa, 2020; Kok, 2003; Hardwick, 2010) PPARa also controls two other oxygen consuming cytplasmic enzymes: SCD2 and ACOX. PPARa is the master regulator of oxidative fermentation of PUFA in the liver. Not coincidentally, animals increase expression of PPARa as winter approaches and as they become torpid. (Chayama, 2019) Also not coincidentally, the PPARa activating fibrate drug fenofibrate increased D6D activity, SCD activity and liver fat in humans. (Oscarrson, 2018)
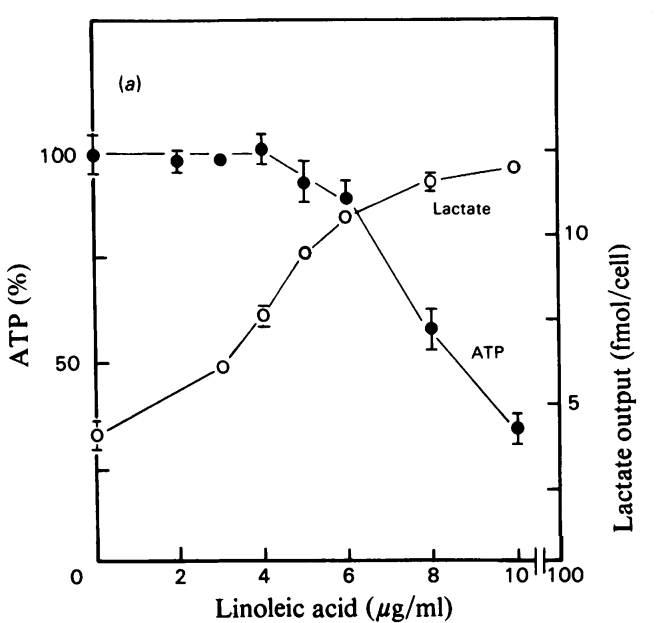
Linoleic acid can power fermentation. As linoleic acid increases to (modern) physiological levels (Pelligrini, 2021) in cell culture, ATP production drops and lactate excretion increases. The cells become glycolytic. The stunning thing about this paper is that as the cells switched to glycolysis, oxygen consumption rates increased! Oxygen is supposed to be burned in the mitochondria for oxidative phosphorylation, not in the cytoplasm for glycolysis.
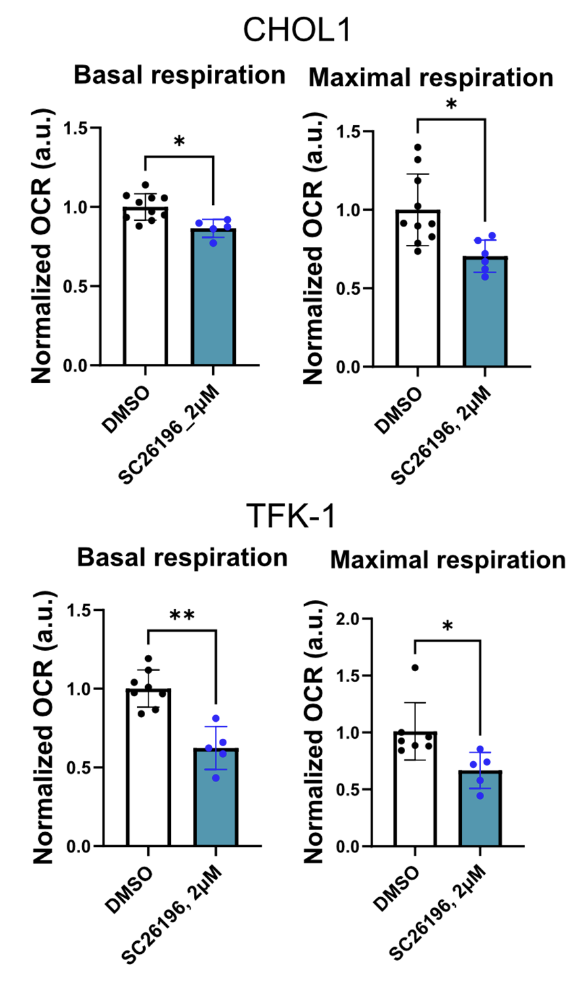
Could the oxygen stealing effects of oxidative fermentation of PUFA be physiologically relevant? How much oxygen can these processes really take? We don’t have a ton of data but the data points we do have are intriguing. Hasegawa showed that when SC26196 – an inhibitor of D6D – was applied to CHOL1 or TFK-1 cancer cells, their basal and maximum oxygen consumption rates dropped by between 20 and 40%. D6D and downstream reactions were using up to 40% of ALL oxygen consumed by the cells. This might actually understate the case since cells in culture are typically grown with very low linoleic acid.
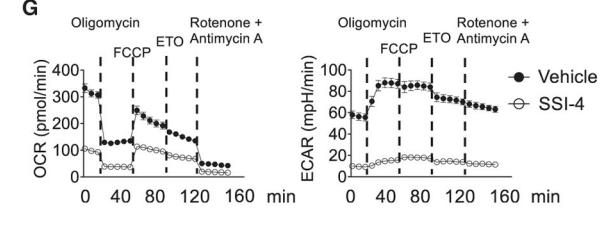
Similarly, in activated B cells, inhibiting SCD1 and SCD2 with SSI-4 decreased oxygen consumption by two thirds while completely inhibiting glycolysis! Activated immune cells are typically highly glycolytic. Ongoing glycolysis requires fermentation to replenish NAD+. This comes from fermenting stearic acid to oleic acid. Stunningly, this fermentation and processes downstream of it consume two thirds of the total oxygen used by the cell. And they’ve been telling you glycolysis doesn’t consume oxygen. Not directly, I suppose.
What can we conclude about this? I am proposing a fundamentally new way to think about the health effects of dietary linoleic and linolenic acids. Both of these fats can be used for oxidative fermentation.
In my opinion, understanding the mechanism is crucial if you want to solve the problem. This looks to me like the primary mechanism by which PUFA effect our metabolisms. Sure, auto-oxidation of PUFA does some collateral damage, but it can’t be the root cause.
Desaturase enzymes can increase oxygen consumption, ie metabolic rate. Is higher metabolic rate always better?
Watch this space coming up, I’m just getting started. I’m going to introduce some scoring algorithms. Not computer algorithms, just “here’s how to think about these things in your head.” I’m going to dust off all of the very interesting feeding experiments done in mice, rats and pigs and see if we can build some rules that make sense of them.
Here’s a rule: PPARa activation is the spark, but linoleic acid is the fuel. Dietary oleic acid provides the spark via OEA, but linoleic acid fuels the fire. So when you combine those two, that’s probably going to go badly. High PUFA lard spiked with soybean oil in diet-induced obesity trials. Another thing that might go badly is if you increase seed oil consumption in an environment with rising environmental PPARa activators such as PFAs from nonstick coatings and phthalates from plastics.
Arslan, P., et al. “Cis -Unsaturated Fatty Acids Uncouple Mitochondria and Stimulate Glycolysis in Intact Lymphocytes.” Biochemical Journal, vol. 217, no. 2, Jan. 1984, pp. 419–25, https://doi.org/10.1042/bj2170419.
Barbier, Olivier, et al. “Peroxisome Proliferator-Activated Receptor α Induces Hepatic Expression of the Human Bile Acid Glucuronidating UDP-Glucuronosyltransferase 2B4 Enzyme.” Journal of Biological Chemistry, vol. 278, no. 35, 2003, pp. 32852–60, https://doi.org/10.1074/jbc.M305361200.
“Peroxisome Proliferator-Activated Receptor α Induces Hepatic Expression of the Human Bile Acid Glucuronidating UDP-Glucuronosyltransferase 2B4 Enzyme.” Journal of Biological Chemistry, vol. 278, no. 35, 2003, pp. 32852–60, https://doi.org/10.1074/jbc.M305361200.
Chayama, Yuichi, et al. “Molecular Basis of White Adipose Tissue Remodeling That Precedes and Coincides With Hibernation in the Syrian Hamster, a Food-Storing Hibernator.” Frontiers in Physiology, vol. 9, Jan. 2019, p. 1973, https://doi.org/10.3389/fphys.2018.01973.
Filadi, Riccardo, et al. “Mitofusin 2 Ablation Increases Endoplasmic Reticulum–Mitochondria Coupling.” Proceedings of the National Academy of Sciences, vol. 112, no. 17, Apr. 2015, https://doi.org/10.1073/pnas.1504880112.
Gao, Huifang, et al. “CYP4A11 Is Involved in the Development of Nonalcoholic Fatty Liver Disease via ROS‑induced Lipid Peroxidation and Inflammation.” International Journal of Molecular Medicine, Jan. 2020, https://doi.org/10.3892/ijmm.2020.4479.
Hardwick, James P., et al. “PPAR/RXR Regulation of Fatty Acid Metabolism and Fatty Acid ω ‐Hydroxylase (CYP4) Isozymes: Implications for Prevention of Lipotoxicity in Fatty Liver Disease.” PPAR Research, edited by John Y. L. Chiang, vol. 2009, no. 1, 2009, p. 952734, https://doi.org/10.1155/2009/952734.
Hasegawa, Kohsei, et al. “Delta‐6 Desaturase FADS2 Is a Tumor‐promoting Factor in Cholangiocarcinoma.” Cancer Science, vol. 115, no. 10, 2024, pp. 3346–57, https://doi.org/10.1111/cas.16306.
Hucik, Barbora, et al. “Reduced Delta-6 Desaturase Activity Partially Protects against High-Fat Diet-Induced Impairment in Whole-Body Glucose Tolerance.” The Journal of Nutritional Biochemistry, vol. 67, 2019, pp. 173–81, https://doi.org/10.1016/j.jnutbio.2019.02.005.
Jansson, P. A., et al. “Lactate Release from the Subcutaneous Tissue in Lean and Obese Men.” Journal of Clinical Investigation, vol. 93, no. 1, Jan. 1994, pp. 240–46, https://doi.org/10.1172/JCI116951.
Kim, Wondong, et al. “Polyunsaturated Fatty Acid Desaturation Is a Mechanism for Glycolytic NAD+ Recycling.” Cell Metabolism, vol. 29, no. 4, 2019, pp. 856-870.e7, https://doi.org/10.1016/j.cmet.2018.12.023.
Kok, Tineke, et al. “Peroxisome Proliferator-Activated Receptor Alpha (PPARalpha)-Mediated Regulation of Multidrug Resistance 2 (Mdr2) Expression and Function in Mice.” Biochemical Journal, vol. 369, no. 3, Feb. 2003, pp. 539–47, https://doi.org/10.1042/bj20020981.
Kröger, Janine, et al. “Erythrocyte Membrane Phospholipid Fatty Acids, Desaturase Activity, and Dietary Fatty Acids in Relation to Risk of Type 2 Diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study.” The American Journal of Clinical Nutrition, vol. 93, no. 1, 2011, pp. 127–42, https://doi.org/10.3945/ajcn.110.005447.
Luis, Géraldine, et al. “Tumor Resistance to Ferroptosis Driven by Stearoyl-CoA Desaturase-1 (SCD1) in Cancer Cells and Fatty Acid Biding Protein-4 (FABP4) in Tumor Microenvironment Promote Tumor Recurrence.” Redox Biology, vol. 43, 2021, p. 102006, https://doi.org/10.1016/j.redox.2021.102006.
Ntambi, James M., et al. “Loss of Stearoyl–CoA Desaturase-1 Function Protects Mice against Adiposity.” Proceedings of the National Academy of Sciences, vol. 99, no. 17, Aug. 2002, pp. 11482–86, https://doi.org/10.1073/pnas.132384699.
O’Neill, Lucas M., et al. “Global Deficiency of Stearoyl-CoA Desaturase-2 Protects against Diet-Induced Adiposity.” Biochemical and Biophysical Research Communications, vol. 527, no. 3, 2020, pp. 589–95, https://doi.org/10.1016/j.bbrc.2020.04.077.
Oscarsson, Jan, et al. “Effects of Free Omega-3 Carboxylic Acids and Fenofibrate on Liver Fat Content in Patients with Hypertriglyceridemia and Non-Alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Study.” Journal of Clinical Lipidology, vol. 12, no. 6, 2018, pp. 1390-1403.e4, https://doi.org/10.1016/j.jacl.2018.08.003.
Pellegrini, Cara N., et al. “Individual Non-Esterified Fatty Acids and Incident Atrial Fibrillation Late in Life.” Heart, vol. 107, no. 22, 2021, pp. 1805–12, https://doi.org/10.1136/heartjnl-2020-317929.
—. “Individual Non-Esterified Fatty Acids and Incident Atrial Fibrillation Late in Life.” Heart, vol. 107, no. 22, 2021, pp. 1805–12, https://doi.org/10.1136/heartjnl-2020-317929.
Wang, Chenxuan, et al. “Inhibition of Δ-6 Desaturase Reduces Fatty Acid Re-Esterification in 3T3-L1 Adipocytes Independent of Changes in N3-PUFA Cellular Content.” Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids, vol. 1867, no. 7, 2022, p. 159160, https://doi.org/10.1016/j.bbalip.2022.159160.
Warensjö, Eva, et al. “Associations between Estimated Fatty Acid Desaturase Activities in Serum Lipids and Adipose Tissue in Humans: Links to Obesity and Insulin Resistance.” Lipids in Health and Disease, vol. 8, no. 1, 2009, p. 37, https://doi.org/10.1186/1476-511X-8-37.
Witt, Anke, et al. “Fatty Acid Desaturase 2 Determines the Lipidomic Landscape and Steroidogenic Function of the Adrenal Gland.” Science Advances, vol. 9, no. 29, July 2023, p. eadf6710, https://doi.org/10.1126/sciadv.adf6710.
Yashiro, Hiroaki, et al. “A Novel Selective Inhibitor of Delta-5 Desaturase Lowers Insulin Resistance and Reduces Body Weight in Diet-Induced Obese C57BL/6J Mice.” PLOS ONE, edited by Marcia B. Aguila, vol. 11, no. 11, Nov. 2016, p. e0166198, https://doi.org/10.1371/journal.pone.0166198.
Zhou, Xian, et al. “Stearoyl-CoA Desaturase-Mediated Monounsaturated Fatty Acid Availability Supports Humoral Immunity.” Cell Reports, vol. 34, no. 1, 2021, p. 108601, https://doi.org/10.1016/j.celrep.2020.108601.

I feel like you are getting to some unified concepts here about how metabolism is affected by different substances. Would be interesting to see if there is a similar effect from bcaa/proteins in terms of oxygen consumption in the cell that lowers available O2 for the mitochondria.
Thank goodness you are back, keep up the good work.
Nice!
Did you ever hear of Dr Barry Sears, of the Zone Diet ( maybe 30’ish year’s ago )? He was big into Eicosanoids.
Apparently lots of new research since then.
Looking forward to what comes next 👍
I’m not a biologist, just a lay person enthusiast who reads and re-reads all of these posts so I can assimilate the theory and chemistry. So this last series had got me thinking on earlier posts back in ’21 and later regarding lactate being elevated in obesity. We’re we’re looking at it from the standpoint of pyruvate- lactate buffering when nadh is high and needing nad+ to break down the pyruvate to acetyl coA. But I’m wondering now if runaway cori cycling of glucose to lactate has compounded this problem: https://www.sciencedirect.com/science/article/pii/S0261561421000868. You can read it all but if in a hurry, skip down to point 6 for the implications.
Here’s what I know n=1. Higher blood glucose during fasted states but will go to normal after eating mixed meal or protein/ fat meal. Indigestion of high sugar foods, particularly at night but even during day. muscle strengrh ok but stamina diminished and if I over do it on a day I get whole body pain like “lactic acid” build up feeling that can disrupt sleep for a day or two. Would it make any sense that low grade pufa fuel, that is now fermenting instead of burning, along with cori cycling of glucose has so imbalanced metabolism that there’s little to no glycogen production and little citric acid cycling leaving a body constantly fatjgued, sore inflamed and anabolic?
Brilliant
Omega 3s compete with 6 for desaturase enzymes as well as elongase. Transgenic mouse (FAT 1/2) models and larger RCT studies demonstrate that the omega 6/3 ratio is important in predicting negative outcomes, even in a non omega -6 dose dependent way. In other words, even at high levels of omega 6, if you’re able to match omega 3 (somehow, despite its dietary lack) it has a protective effect. How could this theory account for this?
Fascinating, thanks.
It seems like there is also an interaction with mitochondrial fusion/fission. It looks like PPARa also promotes fusion. Animal cells need to cycle through fusion and fission to be healthy: fusion mixes the mitochondrial dna; fission allows mitophagy to clean up dysfunctional mtDNA.
I love how you always back up your points with such solid examples.
I’ve been reading your blog, and the biological mechanism behind the fat burning makes sense, but overall, I’m worried about the encouragement of ROS production. Reactive oxygen species are pretty much what we’ve found to be the cause of almost everything regarding aging, “age-related” diseases, brain and protein dysfunctions (prion diseases), and why people die/die early from ROS accumulation in the gut in the case of Fatal Familial Insomnia. It is known that insulin is an anabolic hormone and the increase of insulin serum levels in the blood leads to insulin resistance, and the whole host of diseases attributed to IR-related conditions (higher risks for cardiovascular disease, diabetes, fatty liver disease, et cetera). Could this be why it is often said that anabolism and longevity cannot both be achieved?
The croissant diet seems like an awesome way to stay trim, but even skinny people are susceptible to the accumulation of visceral fat and the complications of that fat’s inflammation. We cite the Chinese as a group who primarily subsisted off of rice and never became obese, but asian groups are especially susceptible to abdominal obesity and liver diseases. Do you think such a croissant diet would be viable long-term without the body at least trying to downregulate ROS by producing anti-oxidants?