In the previous post, we introduced our unlikely hero superoxide, a free radical. We also introduced peroxide and the term “Reactive Oxygen Species” (ROS), the term that refers collectively to superoxide and peroxide and others in the family of pro-oxidant molecules.
We evolved to thrive in the presence of oxygen and ROS after the Great Oxygenation. Every single human cell, and in fact every cell of every organism that can live in the presence of oxygen has a robust inbuilt antioxidant system.
Antioxidant defense starts with Superoxide Dismutase (SOD). It is an enzyme that converts superoxide into peroxide – the stuff that they sell in brown bottles at the drugstore. Superoxide dismutase (SOD) reduces the half life of superoxide dramatically. SOD is a remarkable enzyme. It is the fastest known enzyme, able to convert superoxide to hydrogen peroxide as fast as diffusion will allow. Superoxide in the presence of SOD only exists for tiny fractions of a second. SOD prevents superoxide from causing any real damage. SOD is found in great quantities in the mitochondria, which is the site of the majority of superoxide production in most cells.
Peroxide is still a strong oxidant, but it’s not NEARLY as potent or reactive as superoxide.
Interestingly, Superoxide dismutase is at the forefront of many battles. Immune cells such as macrophages and neutrophils have enzymes like NADPH Oxidase (an enzyme that produces superoxide) attached to their outer membranes. When our immune cells encounter a bacterial cell, those enzymes emit a stream of superoxide to destroy the invading bacteria in a type of watery fire tornado. The bacteria will often release their own version of superoxide dismutase in an attempt to avoid the fire.
The next character in our antioxidant system is glutathione. Glutathione is sometimes called the body’s “master antioxidant”. Glutathione is like a standing army. When peroxide is produced by Superoxide Dismutase, glutathione molecules step up to disarm them. Glutathione acts like a buffer, essentially giving an electron to peroxide, which allows it to be converted to water and Oxygen by an enzyme (Glutathione Peroxidase).
Antioxidants are electron donors.
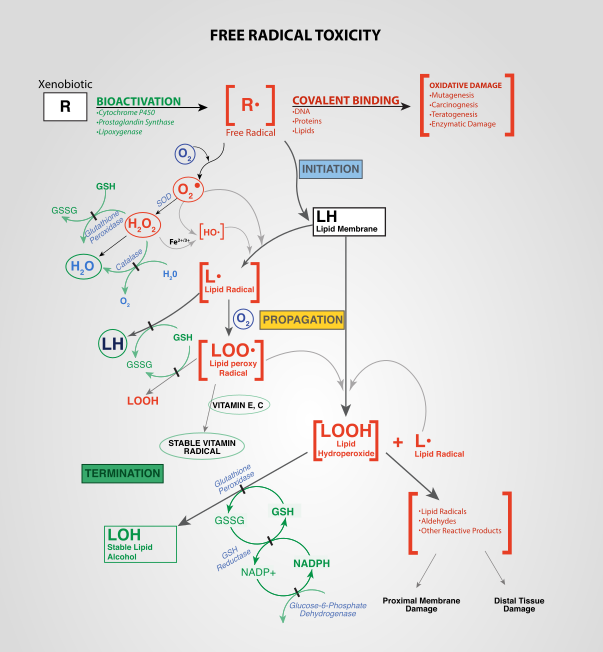
Photo: By Dan Cojocari – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=46529393
After glutathione gives up its electron, it is said to be oxidized. Enzymes then use electron donors to reduce glutathione (Glutathione Reductase) so that it is again ready to donate an electron to another molecule of peroxide. It’s a cycle. In healthy cells at rest there is a large pool of reduced glutathione ready to respond to any burst of oxidants. Glutathione is the buffer that maintains a healthy redox balance in every human cell.
The last character in our antioxidant system is catalase. Much like glutathione, catalase converts peroxide into water and oxygen. Unlike glutathione, catalase doesn’t maintain a standing army of shock troops waiting at the ready for an oxidative event. Catalase is like a slow release valve that slowly and steadily removes the oxidative pressure of peroxide.
