So we’re getting back to why you shouldn’t eat seed oils. If I tried to explain it in a single post, it would be too much. Anabolism vs catabolism. Redox balance. How do cells allocate power to different pathways? What do PUFA have to do with any of this?
If you want understanding you have to put the background work in. Paint the fence. Wax on, wax off.
This article is about the “respiratory burst”, the metabolic switch that immune cells undergo when they are activated by an invader. The become heavily anabolic and glycolytic within minutes to hours. The adipose cells in obesity are glycolytic and anabolic.
This starts off about immunity and ends at PPAR alpha (PPARa), the master controller of PUFA metabolism. For some reason PUFA metabolism is implicated in the respiratory burst.
We get to visit with amphioxus along the way.
Now we’ve leapt forward in time. It is 520MYAish. The Cambrian Explosion has happened.
Cambrian Explosion: There is a distinct boundary line in most sediments. After the Cambrian Explosion (541MYAish to 520MYAish) there are plentiful fossils: trilobytes, brachiopods, bryozoans, etc. In older sediments there are almost none. The explosion was a time of great creativity for the animal clade. The period ended with arthropods(trilobytes), echinoderms (starfish), molluscs and more. Since that time there have been no new body plans.
The Cambrian Explosion was the end of the beginning of the animal clade. End Section.
What is our current clade? We are the chordates. We have a notochord, a stiffened rod which will ultimately give rise to the spine. We look like this 520MYAish fossil from China:
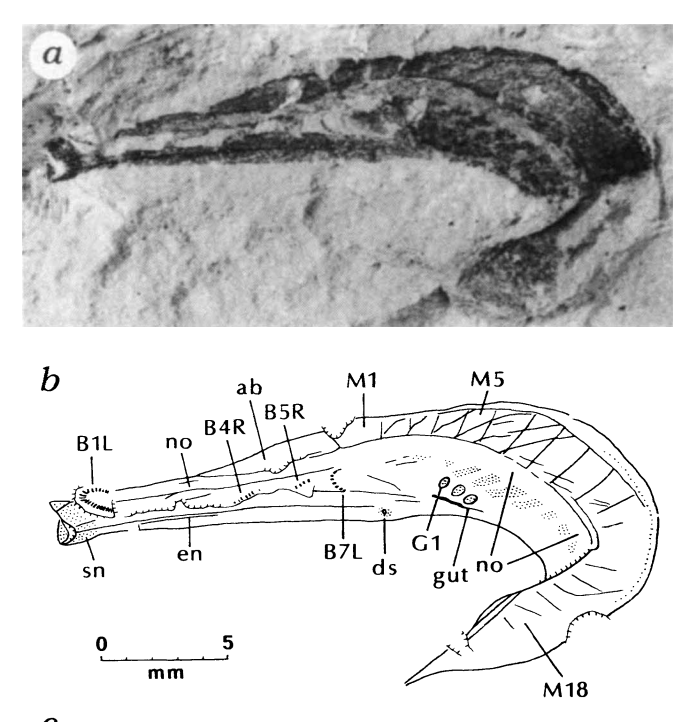
This looks a lot like amphioxus, who is still plentiful in the shallow seas around the globe. They’re easy to catch. They eat them in Japan.

Amphioxus is the most ancient species that has been demonstrated to have true macrophage like cells that are capable of undergoing the respiratory burst. (Yang, 2014) The burst is a response in immune cells to a stimulus such as a bacterial invader. In classical M1 macrophage activation, the cells go from their normal behavior to extraordinary anabolic in a timeframe of minutes to hours. They become heavily glycolytic, just like the adipose cells of obese humans.
Amphioxus has the full complement of characters discussed in the last post who control the hypoxia response: HIF-1a, NF-kB and NRF2.
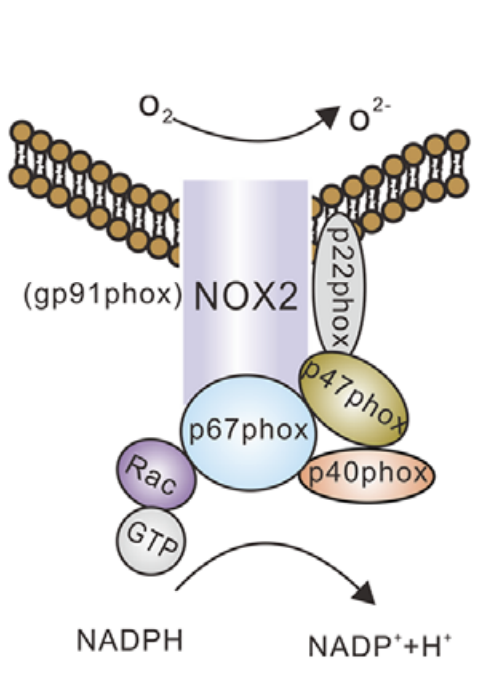
They also contain NOX. NADPH oxidase. NOX are enzymes which span cell membranes. When a macrophage encounters an invader they use NOX to spray a stream of superoxide at it. Superoxide is a potentially damaging reactive oxygen species that is made from extracellular oxygen. NOX enzymes draw their power from NADPH generated in the Pentose Phosphate Pathway (PPP).
ROS from NOX enzymes created by adipose tissue macrophages are implicated in the insulin resistance seen in obesity.
The Onion Of Biology: The reason we are interested in the question of “what was the most ancient thing in our lineage to have…” is that if we want to reverse engineer how the system is designed, we need to know which components were in place first.
A clade is a group of organisms who share a common ancestor. We are not in the clade of amphioxus because we did not evolve from amphioxus. But we share a common ancester with amphioxus who had a notochord and amebocytes who were capable of undergoing the respiratory burst. Amphioxus and us are both in the chordate clade.
When a group of features come together in an organism in a way that is crucial to the survival of the organism those features become locked in and immutable. We say those features are conserved. Amphioxus launches an immune response with NF-kB, HIF-1a, NRF2 and NOX2. So do we. We diverged over a half billion years ago and we’ve both been doing it the same way ever since.
Each layer of the onion represents a clade. Members of the clade tend to do the same things the same way. Earlier diverging clades tend to do them differently. More “evolved” clades add a new layer over the older conserved ones rather than monkeying around in the lower layers. End Section.
Here’s how we do it. Here’s how the response is coordinated between NF-kB, Nrf2 and HIF-1a. This looks an awful lot like the response to hypoxia we saw, except that this time it is being triggered by NF-kB rather than HIF-1a. The cells are becoming glycolytic while oxygen is still plentiful. Glycolytic does not require hypoxia. This response anticipates the hypoxia to come.
You don’t need to memorize this. The most interesting thing is what is AFTER the table.
| Enzyme | Activation window during the respiratory burst | Effect on glycolysis and the PPP |
|---|---|---|
| NF-κB (Kaul, 1996) | 5 – 10 min after PMA/ADP stimulation; rapid p65/p50 entry into the nucleus driven by burst-derived H₂O₂ | Kicks off an early pro-inflammatory metabolic shift: up-regulates GLUT1 and HK2 to raise glycolytic flux and induces PDK1, which phosphorylates and inhibits pyruvate dehydrogenase, thereby restricting pyruvate entry into the TCA cycle. PPP enzymes are not directly targeted; any PPP rise comes indirectly from the larger glucose-6-phosphate pool. |
| Nrf2 (Ishii, 2000) | ≈ 30–45 min—Keap1 oxidation frees Nrf2; nuclear build-up by 45 min. ARE-target mRNAs rise from 1 h and peak near 3 h | Directly boosts oxidative-PPP genes (G6PD, PGD, TKT, TALDO), diverting some glucose-6-phosphate into NADPH production for antioxidant defence and slightly easing glycolytic throughput. |
| HIF-1α (Rius, 2008) | 90–120 min: NF-κB drives new Hif1a mRNA; stabilized protein accumulates ≈ 3–4 h into the burst/hypoxia | Sustains long-term stress metabolism: up-regulates GLUT1, HK2, PFK-1, LDHA and its own set of PDK isoforms, keeping glycolytic flux high while further throttling TCA entry. It also enhances PPP genes such as G6PD, supplying extra NADPH for redox balance. |
PPARa Enhances The Glycolytic Burst
PPARa controls fat oxidation and remodelling of polyunsaturated fats via the desaturase enzymes D6D and D5D. It feels out of place in this context. During the respiratory burst, most available oxygen is converted to superoxide, making an infection site hypoxic. You can’t burn fat in the mitochondria without oxygen. Nor can you generate net NADPH to drive NOX production of ROS from fat. PPARa is also known as anti-inflammatory.
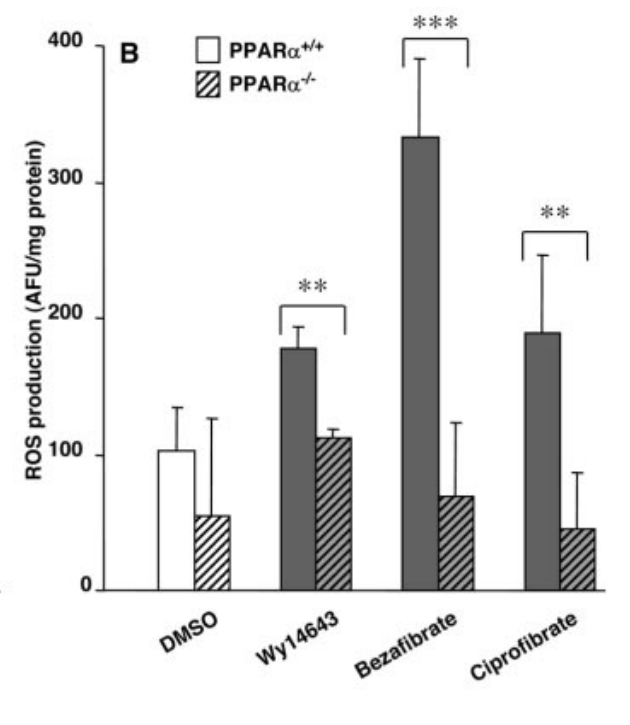
Yet. Macrophages lacking PPARa produce 40% less superoxide during the respiratory burst. (Teissier, 2004) Cells WITH PPARa increase superoxide production up to 300% when PPARa activators Wy1643, bezafibrate or ciprofibrate are added to this mix. These increases are nixed if PPARa isn’t present.
The master enzyme involved in PUFA metabolism is implicated in powering the respiratory burst, the NADPH-driven release of superoxide by NOX2. Why? How? Come back next time.
Chen, J. Y., et al. “A Possible Early Cambrian Chordate.” Nature, vol. 377, no. 6551, 1995, pp. 720–22, https://doi.org/10.1038/377720a0.
Ishii, Tetsuro, et al. “Transcription Factor Nrf2 Coordinately Regulates a Group of Oxidative Stress-Inducible Genes in Macrophages.” Journal of Biological Chemistry, vol. 275, no. 21, 2000, pp. 16023–29, https://doi.org/10.1074/jbc.275.21.16023.
Kaul, Nalini, and Henry Jay Forman. “Activation of NFκB by the Respiratory Burst of Macrophages.” Free Radical Biology and Medicine, vol. 21, no. 3, 1996, pp. 401–05, https://doi.org/10.1016/0891-5849(96)00178-5.
Nascè, Alberto, et al. “NADPH Oxidases Connecting Fatty Liver Disease, Insulin Resistance and Type 2 Diabetes: Current Knowledge and Therapeutic Outlook.” Antioxidants, vol. 11, no. 6, June 2022, p. 1131, https://doi.org/10.3390/antiox11061131.
Rius, Jordi, et al. “NF-ΚB Links Innate Immunity to the Hypoxic Response through Transcriptional Regulation of HIF-1α.” Nature, vol. 453, no. 7196, 2008, pp. 807–11, https://doi.org/10.1038/nature06905.
Teissier, Elisabeth, et al. “Peroxisome Proliferator–Activated Receptor α Induces NADPH Oxidase Activity in Macrophages, Leading to the Generation of LDL with PPAR-α Activation Properties.” Circulation Research, vol. 95, no. 12, Dec. 2004, pp. 1174–82, https://doi.org/10.1161/01.RES.0000150594.95988.45.
Yang, Ping, et al. “Origin of the Phagocytic Respiratory Burst and Its Role in Gut Epithelial Phagocytosis in a Basal Chordate.” Free Radical Biology and Medicine, vol. 70, 2014, pp. 54–67, https://doi.org/10.1016/j.freeradbiomed.2014.02.007.
Yuan, Shaochun, Jie Ruan, et al. “Amphioxus as a Model for Investigating Evolution of the Vertebrate Immune System.” Developmental & Comparative Immunology, vol. 48, no. 2, 2015, pp. 297–305, https://doi.org/10.1016/j.dci.2014.05.004.
Yuan, Shaochun, Jie Zhang, et al. “The Archaic Roles of the Amphioxus NF-ΚB/IκB Complex in Innate Immune Responses.” The Journal of Immunology, vol. 191, no. 3, Aug. 2013, pp. 1220–30, https://doi.org/10.4049/jimmunol.1203527.
