When I took oncology at Cornell in the mid-90’s, the root cause of cancer was explained in the following way. Certain things – such as ionizing radiation from a nuclear explosion – cause free radical formation. The free radicals can damage the DNA, causing genetic mutations. When enough mutations accumulated in key genes affecting the cell cycle – “oncogenes” and “tumor suppressor genes” – the cells would advance to a cancerous state. Free radicals were just simply bad news.
But times change and we learn new things.
Superoxide ( O2•− ) is a free radical. It is a powerful oxidant. Superoxide is molecular Oxygen that has gained an additional unpaired electron (technically it’s been “partially reduced”). As I’ve pointed out before Oxygen is a very reactive molecule because chlorophyll uses the energy of the sun to take it’s electrons away and it would really like them back! But Oxygen’s got nothing on superoxide in that department. Having an additional, unpaired electron actually makes superoxide much, much more reactive than molecular oxygen. Superoxide WILL go out and immediately oxidize something (take its electrons) in less than a second in a typical cell. Superoxide can oxidize proteins, causing them to lose their functionality. It can oxidize polyunsaturated fats, causing them to form dangerous “lipid peroxides”, which are themselves free radicals. And yes, it can cause strand breaks in DNA, leading to genetic mutations.
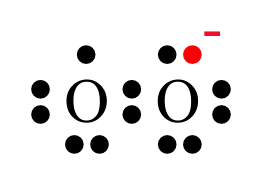
Photo: Public Domain, https://commons.wikimedia.org/w/index.php?curid=784840
And yet I’m calling superoxide a hero. Evolution is very clever. We are oxidative creatures. The fire that burns in our mitochondria creates the superoxide that informs our basic cellular processes. We evolved to use superoxide and its cousin hydrogen peroxide, known collectively as Reactive Oxygen Species (ROS) or simply oxidants, as primary signalling molecules in many of our most basic life processes.
Reactive Oxygen Species (ROS) control the entire cell cycle. Stem cells (baby cells, essentially) are totally protected from ROS. A little bit of ROS causes them to start to grow and move to where they’ll be needed as an adult cell. A little more ROS causes them to differentiate (grow up) and settle into their final role in the body. And at the end of their useful life, a pulse of ROS sends them into “apoptosis” – programmed cell death.
ROS is the signal.
When you eat, ROS in the hypothalamus causes you to feel satiated. When a bacteria invades, ROS tells immune cells to multiply. When immune cells arrive at the scene they destroy the invading bacteria by using enzymes in their outer membrane that produce a jet of superoxide that literally burns the bacteria to death. Fire tornado.
ROS is the signal.
ROS are produced in your mitochondria when you undergo respiration (the oxidation of hydrocarbons), they are produced in your muscle cells when you exercise, they are produced in your skin when you stand in sunlight, they are produced by enzymes that recognize foreign bacteria.
Sure, sometimes superoxide can do damage, but 99% of the time superoxide is an incredibly important signalling molecule and an immune and cancer fighting tool. Evolution is very clever.
In the next entry we’re going to learn how our bodies antioxidant defense system prevents us from getting damage from superoxide and the other ROS. In the following post we’re going to look at 2 scientific papers that shows dramatic real life effects of ROS functioning as a signalling molecule and its interplay with antioxidants.
