Video Summary
The Inuit live in the Arctic on the seal blubber. This would be considered a ketotic diet, yet they are not heavily in ketosis.
The reason is that they have a genetic deficiency in a gene called CPT1 that doesn’t allow them to import long chain fats – normal things such as stearic acid (chocolate) and oleic acid (olive oil) – into their mitochondria. This mutation has some serious disadvantages such as hypoketotic hypoglycaemia, seizers and sudden unexpected death in infancy. (Collins, 2010)
Despite theses serious drawbacks, the frequency of this mutant allele in the Inuit population suggests that there was strong selection pressure for this mutation. One of the effects of the mutation is a lack of ketosis. Ketones are produced in liver mitochondria and without the ability to directly import fat, there seems to be a lack of sufficient Acetyl-CoA to generate significant ketones.
It has been suggested that the reason this mutation was selected for is that being in ketosis long term is problematic. The argument is that in a cold environment, a combination of hypothermia with baseline ketosis could put you into ketoacidosis.
I am putting forth another hypothesis: highly unsaturated fat from seal blubber would put the liver into serious oxidative and reductive stress. Seal blubber is well over 20% long chain omega 3 fats (Kuhnlein, 1991), which are highly prone to oxidation. (Meydani, 1991) Compounding this problem, seal blubber is over 60% MUFA, which creates a high NADH/NAD+ ratio in the cell (AKA reductive stress)(Dziewulska, 2020), which drives the peroxidation of PUFA (Ogura, 2020; Yan, 2021).
Mice who lack the nuclear receptor PPAR alpha are killed via acute liver failure by a diet containing around 25% of calories as fish oil. (Luo, 2021) In normal circumstances, long chain PUFA are broken down in peroxisomes. PPAR alpha is activated by both the MUFA and PUFA in seal blubber. PPAR alpha controls peroxisomal activity, expression of CPT1, and detoxification of xenobiotics (Claudel, 2007). Humans have low peroxisomal activity. (Ammerschlager, 2004)
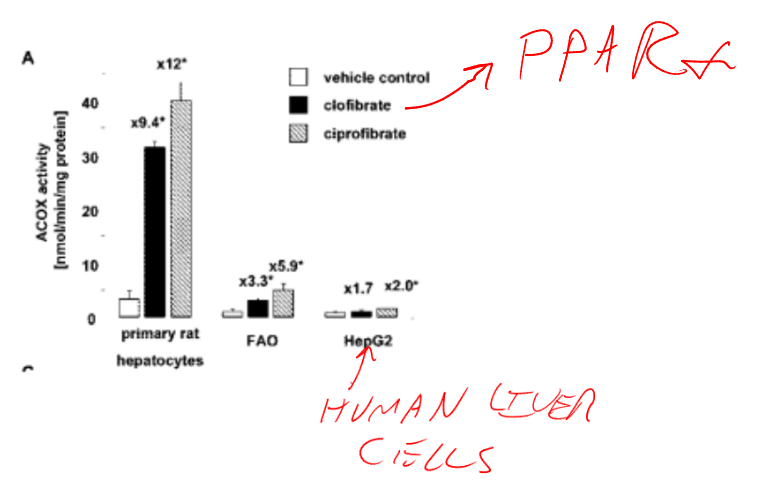
Seal blubber based diets would have provided at least twice the long chain PUFA as the diet that killed mice lacking the ability to increase peroxisomal liver activity.
I am suggesting that by losing CPT1 functionality, the Inuit were able to take advantage of the detoxification and peroxisomal amplifying effects of PPAR alpha activation while avoiding the increase in NADH/NAD+ ratio caused by an increase in CPT1 expression. Increased peroxisomal activity would have increased the rates of elimination of the long chain PUFA. The lower NADH/NAD+ ratios would have helped to maintain the PUFA as PUFA, rather than becoming peroxidized products such as malondialdehyde (MDA) – an indicator of oxidative stress.
Luo et al. recently demonstrated that mice and human liver cells with reduced CPT1a function have hugely reduced MDA levels. The human liver with reduced CPT1 function are a pretty reasonable approximation for what is happening in an Inuit liver. Knocking down CPT1a reduced MDA levels by two thirds.
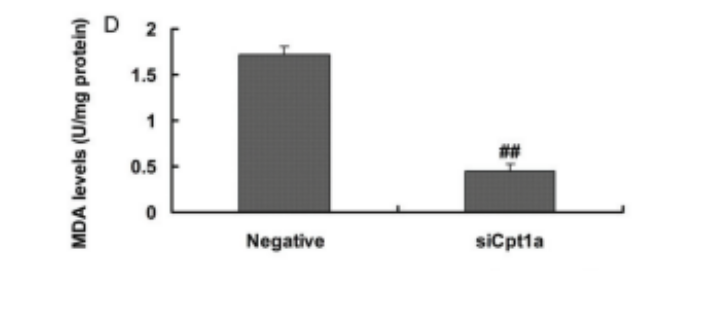
Seal blubber’s ratio of Saturated/MUFA/PUFA is 10/64/26. (Kuhnlein, 1991) I don’t believe fat of this composition is a suitable staple food of humans. This amount of highly-peroxidizable PUFA with MUFA would have led to an apocalypse of liver failure among the earliest arctic inhabitants. Many would have perished just like the mice lacking PPAR alpha on a fish oil diet. Luo et al have demonstrated that dramatically reduced CPT1 function would have been key to surviving the redox apocalypse.
Ammerschlaeger, M. (2004). Characterization of the Species-Specificity of Peroxisome Proliferators in Rat and Human Hepatocytes. In Toxicological Sciences (Vol. 78, Issue 2, pp. 229–240). Oxford University Press (OUP). https://doi.org/10.1093/toxsci/kfh071
Claudel, T., Cretenet, G., Saumet, A., & Gachon, F. (2007). Crosstalk between xenobiotics metabolism and circadian clock. In FEBS Letters (Vol. 581, Issue 19, pp. 3626–3633). Wiley. https://doi.org/10.1016/j.febslet.2007.04.009
Collins, S. A., Sinclair, G., McIntosh, S., Bamforth, F., Thompson, R., Sobol, I., Osborne, G., Corriveau, A., Santos, M., Hanley, B., Greenberg, C. R., Vallance, H., & Arbour, L. (2010). Carnitine palmitoyltransferase 1A (CPT1A) P479L prevalence in live newborns in Yukon, Northwest Territories, and Nunavut. In Molecular Genetics and Metabolism (Vol. 101, Issues 2–3, pp. 200–204). Elsevier BV. https://doi.org/10.1016/j.ymgme.2010.07.013
Dziewulska, A., Dobosz, A. M., Dobrzyn, A., Smolinska, A., Kolczynska, K., Ntambi, J. M., & Dobrzyn, P. (2019). SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. In Journal of Cellular Physiology (Vol. 235, Issue 2, pp. 1129–1140). Wiley. https://doi.org/10.1002/jcp.29026
Gonçalves-de-Albuquerque, C. F., Medeiros-de-Moraes, I. M., Oliveira, F. M. de J., Burth, P., Bozza, P. T., Castro Faria, M. V., Silva, A. R., & Castro-Faria-Neto, H. C. de. (2016). Omega-9 Oleic Acid Induces Fatty Acid Oxidation and Decreases Organ Dysfunction and Mortality in Experimental Sepsis. In F. Gallyas (Ed.), PLOS ONE (Vol. 11, Issue 4, p. e0153607). Public Library of Science (PLoS). https://doi.org/10.1371/journal.pone.0153607
Greenberg, C. R., Dilling, L. A., Thompson, G. R., Seargeant, L. E., Haworth, J. C., Phillips, S., Chan, A., Vallance, H. D., Waters, P. J., Sinclair, G., Lillquist, Y., Wanders, R. J. A., & Olpin, S. E. (2009). The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations. In Molecular Genetics and Metabolism (Vol. 96, Issue 4, pp. 201–207). Elsevier BV. https://doi.org/10.1016/j.ymgme.2008.12.018
Jiao, H., Ye, P., & Zhao, B. (2003). Protective effects of green tea polyphenols on human HepG2 cells against oxidative damage of fenofibrate. In Free Radical Biology and Medicine (Vol. 35, Issue 9, pp. 1121–1128). Elsevier BV. https://doi.org/10.1016/s0891-5849(03)00506-9
Luo, X., Sun, D., Wang, Y., Zhang, F., & Wang, Y. (2021). Cpt1a promoted ROS-induced oxidative stress and inflammation in liver injury via the Nrf2/HO-1 and NLRP3 inflammasome signaling pathway. In Canadian Journal of Physiology and Pharmacology (Vol. 99, Issue 5, pp. 468–477). Canadian Science Publishing. https://doi.org/10.1139/cjpp-2020-0165
Kuhnlein, H. V., Kubow, S., & Soueida, R. (1991). Lipid components of traditional inuit foods and diets of Baffin Island. In Journal of Food Composition and Analysis (Vol. 4, Issue 3, pp. 227–236). Elsevier BV. https://doi.org/10.1016/0889-1575(91)90034-4
Meydani, M., Natiello, F., Goldin, B., Free, N., Woods, M., Schaefer, E., Blumberg, J. B., & Gorbach, S. L. (1991). Effect of Long-Term Fish Oil Supplementation on Vitamin E Status and Lipid Peroxidation in Women. In The Journal of Nutrition (Vol. 121, Issue 4, pp. 484–491). Elsevier BV. https://doi.org/10.1093/jn/121.4.484
Odgaard, U. (2005). Hearth and home of the Palaeo-Eskimos. In Études/Inuit/Studies (Vol. 27, Issues 1–2, pp. 349–374). Consortium Erudit. https://doi.org/10.7202/010808ar
Ogura, Y., Kitada, M., Xu, J., Monno, I., & Koya, D. (2020). CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. In Aging (Vol. 12, Issue 12, pp. 11325–11336). Impact Journals, LLC. https://doi.org/10.18632/aging.103410
Pawlak, M., Baugé, E., Lalloyer, F., Lefebvre, P., & Staels, B. (2015). Ketone Body Therapy Protects From Lipotoxicity and Acute Liver Failure Upon Pparα Deficiency. In Molecular Endocrinology (Vol. 29, Issue 8, pp. 1134–1143). The Endocrine Society. https://doi.org/10.1210/me.2014-1383
Schrader, M., Costello, J., Godinho, L. F., & Islinger, M. (2015). Peroxisome-mitochondria interplay and disease. In Journal of Inherited Metabolic Disease (Vol. 38, Issue 4, pp. 681–702). Wiley. https://doi.org/10.1007/s10545-015-9819-7
Shin, M., Iwamoto, N., Yamashita, M., Sano, K., & Umezawa, C. (1998). Pyridine Nucleotide Levels in Liver of Rats Fed Clofibrate- or Pyrazinamide-Containing Diets. In Biochemical Pharmacology (Vol. 55, Issue 3, pp. 367–371). Elsevier BV. https://doi.org/10.1016/s0006-2952(97)00507-8
Todisco, S., Santarsiero, A., Convertini, P., De Stefano, G., Gilio, M., Iacobazzi, V., & Infantino, V. (2022). PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). In Biology (Vol. 11, Issue 5, p. 792). MDPI AG. https://doi.org/10.3390/biology11050792
Tsuduki, T., Honma, T., Nakagawa, K., Ikeda, I., & Miyazawa, T. (2011). Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. In Nutrition (Vol. 27, Issue 3, pp. 334–337). Elsevier BV. https://doi.org/10.1016/j.nut.2010.05.017
Varela-Lopez, A., Pérez-López, M. P., Ramirez-Tortosa, C. L., Battino, M., Granados-Principal, S., Ramirez-Tortosa, M. del C., Ochoa, J. J., Vera-Ramirez, L., Giampieri, F., & Quiles, J. L. (2018). Gene pathways associated with mitochondrial function, oxidative stress and telomere length are differentially expressed in the liver of rats fed lifelong on virgin olive, sunflower or fish oils. In The Journal of Nutritional Biochemistry (Vol. 52, pp. 36–44). Elsevier BV. https://doi.org/10.1016/j.jnutbio.2017.09.007
Vukšić, A., Rašić, D., Žunec, S., Božina, T., Konjevoda, P., Lovrić, J., Bilušić, M., & Bradamante, V. (2023). The effects of simvastatin and fenofibrate on malondialdehyde and reduced glutathione concentrations in the plasma, liver, and brain of normolipidaemic and hyperlipidaemic rats. In Archives of Industrial Hygiene and Toxicology (Vol. 74, Issue 1, pp. 34–41). Walter de Gruyter GmbH. https://doi.org/10.2478/aiht-2023-74-3697
Yan, L.-J. (2021). NADH/NAD+ Redox Imbalance and Diabetic Kidney Disease. In Biomolecules (Vol. 11, Issue 5, p. 730). MDPI AG. https://doi.org/10.3390/biom11050730
Rezaei Zonooz, S., Hasani, M., Morvaridzadeh, M., Beatriz Pizarro, A., Heydari, H., Yosaee, S., Rezamand, G., & Heshmati, J. (2021). Effect of alpha-lipoic acid on oxidative stress parameters: A systematic review and meta-analysis. In Journal of Functional Foods (Vol. 87, p. 104774). Elsevier BV. https://doi.org/10.1016/j.jff.2021.104774
