In the introductory series, we first saw an example of ROS (Reactive Oxygen Species) as a signal in a small worm (nematode) called c. elegans. When glucose oxidation in c. elegans is blocked, the worms shift to burning fat which creates more ROS which ultimately leads to a longer lifespan for the worm. Why does fat metabolism generate more ROS than glucose metabolism?
The answer is a really elegant example of evolution. Built in to the electron transport chain in the inner wall of the mitochondria is a bottleneck. As we saw, in the mitochondrial Electron Transport Chain (ETC), both complex I and Complex II pass electrons to Coenzyme Q, which passes them to Complex III. If lots of electrons are coming in through Complex I AND Complex II, the ability of Coenzyme Q to carry electrons is simply overwhelmed. The result is that the electrons have nowhere to go and they ping backwards out of the system – this is known as Reverse Electron Transport (RET) – through complex I and end up being absorbed by Oxygen to create our hero Superoxide.
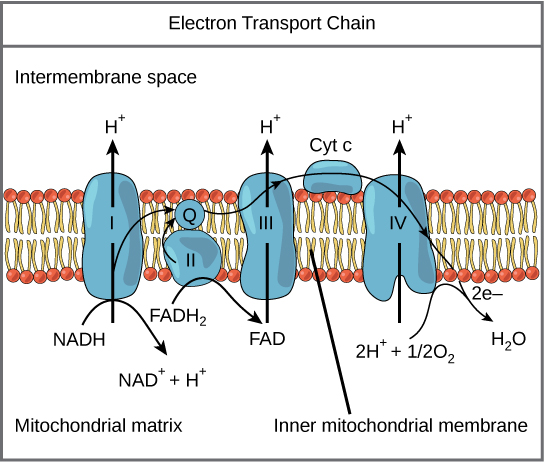
By CNX OpenStax – http://cnx.org/contents/GFy_h8cu@10.53:rZudN6XP@2/Introduction, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=49924807
The production of superoxide in the mitochondria is not an evolutionary mistake!! This is a highly conserved feature of all Eukaryotes (organisms that contain a nucleus and mitochondria). The electron transport chain is designed the same way in yeast as it is in humans and we have already seen the biological effects of the Coenzyme Q bottleneck in worms.
When a molecule of glucose is fully oxidized it produces 10 NADH molecules and 2 FADH2 molecules, a ratio of 5:1 in favor of NADH. When a molecule of palmitic acid – a long chain saturated fat – is fully oxidized it produces 31 NADH molecules and 15 FADH2, a ratio of about 2:1 in favor of NADH. Which is to say that burning saturated fat produces a lot more FADH2 per molecule of NADH than burning glucose. And so long chain saturated fats send the most electrons through Complex II, which causes the most electrons to bottleneck at Coenzyme Q and ping back via Reverse Electron Transport through Complex I and create superoxide.
The Coenzyme Q bottleneck drives ROS production. In c. elegans, that signal affects transcription factors which ends up in increased life of the worm. In large, complex animals like mice and humans (as opposed to yeast and c. elegans), ROS causes physiological insulin resistance.
Saturated fat causes physiological insulin resistance.
