Let’s think about what happens in response to a starchy meal. Blood sugar rises and insulin is released. Insulin attaches to the insulin receptors on the cell membranes of muscle cells, fat cells, etc. The cells respond by moving a glucose transporter, GLUT4, into the cell membrane so that they can start importing glucose. Insulin is the signal that says starch is plentiful, we should switch from fat-burning mode to fat-storing mode. Muscles use this time to build their glycogen reserves – glucose stored in the muscle that they use as fuel. Fat cells stop doing lipolysis – the process of releasing fats into the bloodstream as Free Fatty Acids (FFA) for other cells to use as energy – and they start taking in glucose to do de novo lipogenesis – the production of fat from glucose.
At some point each cell has to make decision that it’s had enough and it needs to stop taking in glucose. After a meal, insulin sensitive cells are taking in blood glucose and using it to pump protons in the mitochondrial membrane to create ATP. At first this is easy. Since you haven’t eaten for a while, lots of ATP has been used and is now available as ADP which the mitochondria can turn back into ATP. The charge gradient across the mitochondrial membrane is low. Electrons run freely through the electron transport chain, protons are pumped and ATP is produced. After a time, much of the ADP has been converted to ATP. This causes the gradient across the mitochondrial inner membrane to increase because protons are still being pumped. The more highly charged the “battery” is at the mitochondrial membrane, the harder it is to push even more protons across the voltage gradient. At this point, electrons will start to ping back out of the electron transport chain. This creates superoxide, which is turned into Hydrogen Peroxide, which migrates out of the mitochondrial membrane and shuts down insulin signalling, making the cell insulin resistant. After the cell becomes insulin resistant, it will stop taking in glucose. It’s full! ROS is the signal.
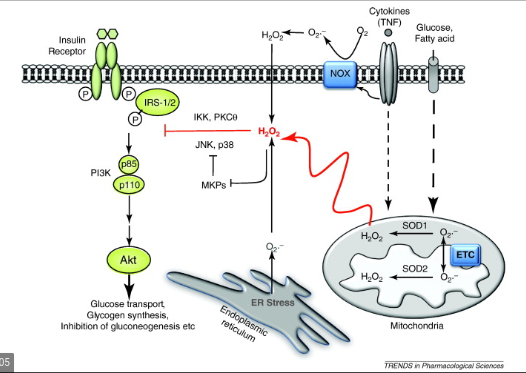
This is “physiological” insulin resistance. It’s not something to be scared of, it happens after every meal. It is an individual cell’s way of deciding to get up from the dinner table.
After a time, the ATP will be used by the cell to do what it needs to do, creating more ADP which will allow the mitochondria to use those extra protons and the gradient across the mitochondrial membrane will drop. Electrons can now move freely through the electron transport chain, ROS production drops and the cell becomes insulin sensitive again.
Physiological Insulin Resistance is the switch that allows each cell to control its energy intake. ROS flips the switch.
I’d like to again thank Peter at Hyperlipid. Imitation is, of course, the sincerest form of flattery. I’ve stolen most of his ideas in this thread with the idea to make them more approachable to most readers but also to extend some of his ideas to new contexts, such as in Chinese peasants eating a high starch diet. Peter is a dedicated low carb dieter and he’ll probably be appalled that I’m using his theory to explain why high starch diets cause leanness. We’ll see! I’ve also taken the liberty of renaming his “protons” theory the ROS Theory of Obesity.

Please write about MUFAs.
Ordinary people like me struggle to understand whether coconut oil is as useful as saturated fat or not. I believe saturated fat is healthy, but can’t eat too much of it, as it upsets my stomach beyond a certain amount, and need more fat in my diet.
MUFAs – are they so bad? In English, for non science brains…
MUFAs are an interesting thing. They’re the middle ground between the pro saturated fat crowd and the mainstream. Everyone is more or less comfortable with them. But if you read this post you can see that the mice given a diet of MUFA had more fat mass than mice given saturated fat OR mice on a low fat control diet. I think that MUFA lacks some of the (for a future post) downsides of PUFA from a fat oxidation perspective, so in that sense I think MUFA is a much safer bet than PUFA. But if you’re struggling with your weight, you might want to avoid MUFA.
This is backed up in this post.