For the background material on understanding this post, start with Hydrogen Peroxide Flips The Switch.
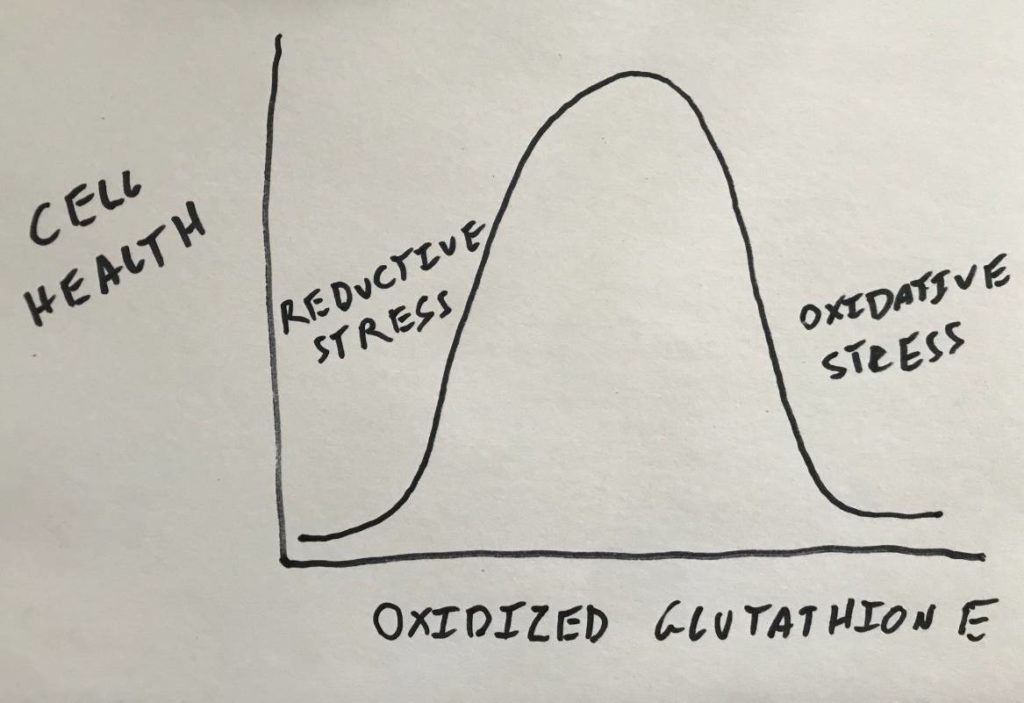
In most cells, most of the time, it is ideal to be in a state of oxidative balance. The simplest measure for this is the percentage of a small molecule called glutathione, sometimes referred to as the body’s “master antioxidant”, that is oxidized.
Hydrogen peroxide is the most important biological oxidant in many cases. It comes in from the mitochondria and other sources such as the endoplasmic reticulum and certain enzymes. It is removed by an enzyme called catalase, which converts the peroxide into water and O2, molecular oxygen. It is also removed by a standing army of glutathione that the cell keeps on hand. This standing army acts like a buffer. A molecule of glutathione can step in and give an electron (antioxidants are electron donors) to hydrogen peroxide, using the enzyme glutathione peroxidase to convert the peroxide into two molecules of water.
Glutathione is oxidized in the process of defusing (reducing) a molecule of hydrogen peroxide. To be able to defuse another hydrogen peroxide, it has to itself be reduced by another enzyme, glutathione reductase, which takes an electron from NADPH, which is oxidized to NADP+ in the process. NADP+ can’t be used to reduce another glutathione until it is reduced. NADPH and reduced glutathione is like a bucket brigade that carries electrons to hydrogen peroxide so that it can be safely turned back into water.
The standing army of glutathione acts like a buffer. To think about how a cell in redox balance works, let’s think again about the insulin receptor we talked about in the last post. When insulin binds its receptor, NADPH oxidase puts out a burst of superoxide, which is quickly converted to hydrogen peroxide by superoxide dismutase. This hydrogen peroxide oxidizes a cysteine residue in the active site of tyrosine phosphatase 1B, which allows the insulin receptor to turn on. The NADPH oxidase puts out an appropriate amount of superoxide to overwhelm the amount of reduced glutathione in close proximity to the insulin receptor. After the burst is done the hydrogen peroxide will soon be removed by other glutathione in the cell as it diffuses away from the site. Normalcy returns.
But what if all of the glutathione is already oxidized? This is a situation in the cell known as “oxidative stress”. There are different ways to define oxidative stress but in this context I’ll define in it as lack of reduced glutathione. In that scenario of oxidative stress, hydrogen peroxide will build up in the cell. When insulin comes to signal it likely won’t make any difference. Tyrosine phosphatase 1B will already be oxidized and thus the insulin receptor will be active all of the time.
And if there is lots of glutathione around and it’s all reduced? Now you have the opposite situation: reductive stress. In this context I’ll define reductive stress as too much reduced glutathione. In this scenario, insulin binds its receptor, NADPH oxidase puts out a burst of ROS, but it is immediately reduced into water. The cysteine residue in the phosphatase is never oxidized and therefore insulin cannot signal.
So having too much oxidized glutathione and having too much reduced glutathione both result in a pathological outcome. What cells need is balance.
Nrf2, Keap1 and AREs
The way that cells keep in oxidative balance is a beautiful biological control system. Nrf2 is what is known as a transcription factor. It’s job is to go into the nucleus and bind the promoters of genes that match a specific sequence. When Nrf2 binds, that gene is produced. The sequence that Nrf2 binds to is known as the Antioxidant Response Element (ARE). Most of the genes that are upregulated by Nrf2 will increase the cells antioxidant capacity, pulling the cell away from oxidative stress and towards reductive stress.
Keap1 inhibits Nrf2 by binding to it and marking it for degradation. Nrf2 is produced continuously, but as long as Keap1 is activated it will be rapidly broken down. Guess what turns off Keap1? That’s right, oxidation of a redox-sensitive cysteine! If you guessed correctly, you’ve been paying attention
When enough glutathione is oxidized, hydrogen peroxide levels build in the cell. the increased hydrogen peroxide oxidizes Keap1, which releases Nrf2 to go into the nucleus to turn on genes that will increase the cells antioxidant capacity.
When the antioxidant capacity is increased, Keap1 will be reduced, Nrf2 will be inhibited and the antioxidant building machinery will be turned off.
This is a very simple and direct feedback loop that showcases the power of redox signalling. The things that makes it beautiful are its directness and simplicity. Hydrogen peroxide is produced directly in the mitochondria, in other organelles and by enzymes. (OK, well, as superoxide first…) The hydrogen peroxide then directly interacts with the the cysteine subunit of Keap1 – the switch that turns antioxidant production on and off. The antioxidant system triggered by Nrf2 is actually quite sophisticated and a lot more complicated than I’ve suggested. But the control loop is simple.
Real World Effects
In recent decades the idea that we should be taken large amounts of antioxidants became trendy. Many large scale clinical trials were done on the effects of supplementing vitamin E, for instance. Every trial increasing dietary antioxidants failed. If you understand the control mechanism, the reason why should be obvious. In the best case scenario, the cells would respond to the increased antioxidants by reducing their own production, leading the cell back to oxidative balance and the effect of the dietary antioxidants would be effectively zero.
But if you take too much of an antioxidant like vitamin E, it could actually push your cells into a state of reductive stress! Basic systems might fail to signal. This is bad.
Remember, we are oxidative vessels. We are fire in a bottle. Taking antioxidant supplements quenches that fire.

It’s almost like physiology is complex.
Great explanation now I’m going to go back and read the previous one again.
Several years ago I did some research into gray hair. One of the discoveries researchers made was that in some cases, the hair actually came in the right color but was ‘bleached’ at the root by a buildup of excess hydrogen peroxide. For whatever reason, there wasn’t enough catalase around to keep it in balance. If we assume the problem is not insufficient catalase production, what would be the probable cause of the build up of h2o2?