I argued in part II of this series that consumption of unsaturated fats fails to create a properly oxygenated fuel mix, which leads to a rise in mitochondrial acetyl-CoA and NADH levels along with a drop in NAD+ levels. This is reductive stress.
The system that replenishes NAD+ while burning fat is the production and reduction of superoxide driven by the activity of three enzymes: succinate dehydrogenase (SDH), Acyl-Coa dehydrogenase and NNT. Interestingly, all three of those enzymes are among the 40 most heavily acetylated proteins (in the heart).1 Acyl-CoA dehydrogenase has been shown to be hyperacetylated in Sirt3 knockout mice (a model of reductive stress) and partially accounts for their low rate of fat oxidation.2 SDH has low activity in both obese humans3 and hibernating mammals4 and has been recently shown to be hyperacetylated in heart disease.5 SDH activity is under the control of Sirt3 and therefore mitochondrial NAD+ levels6. NNT has been shown to be hyperacetylated in aging mouse liver7. The hyperacetylation of NNT can be partially reversed by NAD+ precursor supplementation.
Rising acetylation levels turn off the very enzymes that can replenish NAD+ and activate Sirt3, keeping proteins deacetylated.
Acetylation turns lipogenesis ON
Acetylation turns enzymes involved in fat burning OFF, but in the case of lipogenic transcription factors – both SREBP-1c8 and PPAR gamma9 – acetylation turns them ON!
SREBP-1c is the primary transcription factor that controls the expression of the gene fatty acid synthase, which as the name implies is the enzyme that builds new fat from acetyl-CoA.10 When acetyl-CoA levels rise, SREBP-1c becomes acetylated and activated.8 If NAD+ levels are low, Sirt1 will not be able to deacetylate SREBP-1c to inhibit it and fatty acid synthase production will increase.
Fatty acid synthase levels are increased by reductive stress: high levels of acetyl-CoA and low levels of NAD+.
Dietary unsaturated fats stimulate lipogenesis compared to saturated fats.
If seed oils really cause reductive stress it should be easy to demonstrate in animal models that they increase fatty acid synthase compared to saturated fat.
Let’s start with rats.11 I like this experiment because the saturated fat chosen is beef tallow (probably from kidney fat AKA suet based on fat composition) and because they did an isocaloric study. Beef suet is the most saturated of any natural fat that does not have medium chain triglycerides (MCTs). I don’t have a problem with MCTs really, but they introduce another variable. The fact that they did an isocaloric version of the study eliminates another variable. So here we are comparing apples to apples: all long chain fats at different saturation levels but the same number of calories. I also like that the study used a largely fat free diet except for the supplemental fats and that the fats were 40% of calories, similar to the average American diet. It’s a pretty good study. It’s worth pointing out that this study was done in Spain where perhaps there is less of a stigma against saturated fat.
The study fed rats diets supplemented with – in order of most to least saturated: beef tallow, palm oil, olive oil or sunflower oil. In case I was not clear, the diets were fed isocalorically. The added fats were:
So what happened?
| Beef Tallow | Palm Oil | Olive Oil | Sunflower Oil | |
| Final Weight (g) | 315 | 338 | 331 | 340 |
| Weight Gain (g) | 72 | 94 | 88 | 96 |
| Liver Triglycerides (g/liver) | 67.0 | 86.7 | 108.5 | 90.5 |
| Liver Lipogenic Enzymes | ||||
| Fatty Acid Synthase | 47 | 64 | 111 | 68 |
| Malic Enzyme | 78 | 98 | 145 | 117 |
| Acetyl-CoA carboxylase | 5.2 | 5.7 | 11.7 | 6.2 |
Beef tallow produced significantly lower fatty acid synthase expression than any of the vegetable oils, lower weight gain and less liver fat. Olive oil fared the worst, producing the highest lipogenic gene expression and the most liver fat. Soybean oil did the second worst and palm oil did the third worst.
Pigs
Maybe we should look in a mammal more similar to humans. Pigs have similar sized organs, are similarly prone to obesity and some of them can even get human style heart disease.
P. Duran-Montge did a series of experiments on pigs using beef tallow, sunflower oil or high-oleic sunflower oil added as 10% by weight to a diet that already contained fat from barley, resulting in a diet of around 25% of calories from fat. I wish they had used more fat, but the choice of fat sources and the fact that this experiment was replicated12,13 with similar results makes me feel like this is a well done experiment. I also like that lipogenic gene expression was measured in liver AND adipose tissue and that they measured SREBP-1c as well as fatty acid synthase. Once again, this is a Spanish study. Americans don’t study beef tallow.
In this table I’ve collected data from multiple studies12–14 and I’m reporting lipogenic gene expression relative to beef tallow.
| Beef Tallow | High Oleic Sunflower Oil | Sunflower Oil | |
| Body Fat % | 22.9 | 26.7 | 26.7 |
| Visceral Fat Content | 16.8 | 20.3 | 20.6 |
| Liver Fat (mg/g) | 35.7 | 37.0 | 38.0 |
| Lipogenic Ezymes (Liver) | |||
| Acetyl-CoA carboxylase | 1.00 | 1.49 | 1.16 |
| Fatty Acid Synthase | 1.00 | 1.60 | 1.67 |
| SCD1 | 1.00 | 1.85 | 1.42 |
| SREBP-1c | 1.00 | 1.57 | 1.16 |
| D6D | 1.00 | 1.00 | 1.32 |
| Lipogenic Enzymes (Adipose) | |||
| Acetyl-CoA carboxylase | 1.00 | 1.02 | 1.29 |
| Fatty Acid Synthase | 1.00 | 1.3 | 1.77 |
| SCD1 | 1.00 | 0.95 | 1.26 |
| SREBP-1c | 1.00 | 1.13 | 1.12 |
| D6D | 1.00 | 1.09 | 0.98 |
You can see that in both adipose tissue and the liver, in pigs fed sunflower oil, the levels of fatty acid synthase are higher even though SREBP-1c levels are unchanged. This suggests that something has turned on SREBP-1c. More than likely SREBP-1c has become acetylated. The sunflower oil has created reductive stress compared to the beef tallow.
Low-PUFA Lard
Knowing the effect that unsaturated fats have on lipogenic enzyme expression, I started Firebrand Meats, with the goal of making pork as low in polyunsaturates as possible. In the latest round of testing I sent samples of my lard and some fat I rendered from Smithfield bacon. Look at the tables above and you tell me which lard is more likely to raise the expression of fatty acid synthase. The pigs are purebred berkshire fed a very low fat diet, meaning that the meat is also delicious! Deep red and well marbled, berkshire makes the famed kurobuta pork of Japanese fame. This is really as good as it gets.
Alpha Lipoic Acid
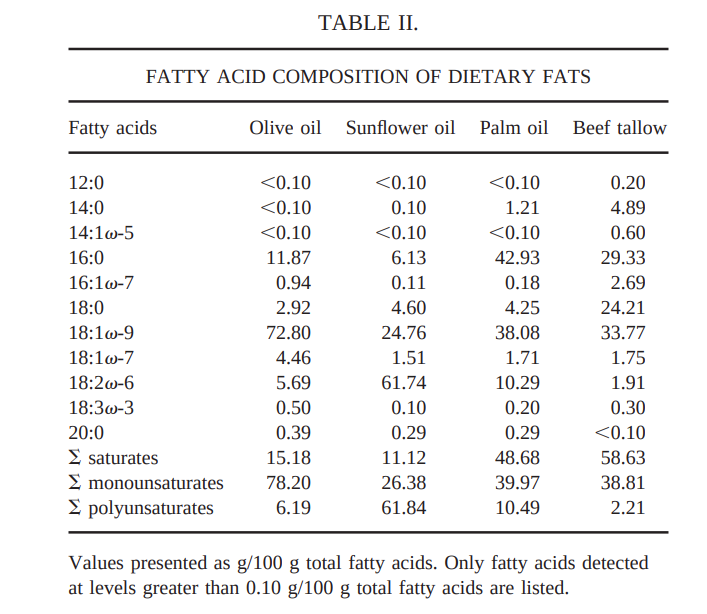
How do we KNOW the change in fatty acid synthase is caused by reductive stress, though? Perhaps there’s some other factor at work that we haven’t considered? If you’ve been reading this series of posts, you already know where I’m going. Alpha lipoic acid is an oxidant that oxidizes NADH to NAD+, reversing reductive stress. If an increase in fatty acid synthase is caused by reductive stress, then alpha lipoic acid should reduce fatty acid synthase expression.
Here is a study in rat liver, the very tissue in which sunflower oil increased fatty acid synthase in the first experiment. What do we find?15
Sunflower oil causes reductive stress and an increase in fatty acid synthase. Lipoic acid eliminates reductive stress and lowers fatty acid synthase.
Conclusions
The lipogenic enzyme fatty acid synthase is an indirect indicator of reductive stress. It is regulated by SREBP-1c, which in turn is regulated by acetyl-CoA and NAD+ levels. Studies in multiple tissues of multiple organisms have shown an upregulation of fatty acid synthase in response to sunflower oil (62% of calories from linoleic acid PUFA) compared to beef tallow (2% of calories from linoleic acid PUFA). This upregulation happens independently of SREBP-1c mRNA levels, suggesting that SREBP-1c is activated by a post-translational modification. SREBP-1c is known to be activated by acetylation and suppressed by Sirt3.
- 1.Foster DB, Liu T, Rucker J, et al. The Cardiac Acetyl-Lysine Proteome. Casarini DE, ed. PLoS ONE. Published online July 2, 2013:e67513. doi:10.1371/journal.pone.0067513
- 2.Bharathi SS, Zhang Y, Mohsen AW, et al. Sirtuin 3 (SIRT3) Protein Regulates Long-chain Acyl-CoA Dehydrogenase by Deacetylating Conserved Lysines Near the Active Site. Journal of Biological Chemistry. Published online November 2013:33837-33847. doi:10.1074/jbc.m113.510354
- 3.Ngo DTM, Sverdlov AL, Karki S, et al. Oxidative modifications of mitochondrial complex II are associated with insulin resistance of visceral fat in obesity. American Journal of Physiology-Endocrinology and Metabolism. Published online February 1, 2019:E168-E177. doi:10.1152/ajpendo.00227.2018
- 4.Armstrong C, Staples JF. The role of succinate dehydrogenase and oxaloacetate in metabolic suppression during hibernation and arousal. J Comp Physiol B. Published online January 30, 2010:775-783. doi:10.1007/s00360-010-0444-3
- 5.Horton JL, Martin OJ, Lai L, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. Published online February 25, 2016. doi:10.1172/jci.insight.84897
- 6.Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of Succinate Dehydrogenase Activity by SIRT3 in Mammalian Mitochondria. Biochemistry. Published online December 18, 2009:304-311. doi:10.1021/bi901627u
- 7.Luo C, Ding W, Zhu S, Chen Y, Liu X, Deng H. Nicotinamide Mononucleotide Administration Amends Protein Acetylome of Aged Mouse Liver. Cells. Published online May 16, 2022:1654. doi:10.3390/cells11101654
- 8.Ponugoti B, Kim DH, Xiao Z, et al. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism*. Journal of Biological Chemistry. Published online October 2010:33959-33970. doi:10.1074/jbc.m110.122978
- 9.Jiang X, Ye X, Guo W, Lu H, Gao Z. Inhibition of HDAC3 promotes ligand-independent PPARγ activation by protein acetylation. Journal of Molecular Endocrinology. Published online June 30, 2014:191-200. doi:10.1530/jme-14-0066
- 10.Griffin M, Sul HS. Insulin Regulation of Fatty Acid Synthase Gene Transcription: Roles of USF and SREBP-1c. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life). Published online October 1, 2004:595-600. doi:10.1080/15216540400022474
- 11.Portillo MP, Chávarri M, Durán D, Rodrı́guez VM, Macarulla MT. Differential effects of diets that provide different lipid sources on hepatic lipogenic activities in rats under ad libitum or restricted feeding. Nutrition. Published online June 2001:467-473. doi:10.1016/s0899-9007(01)00513-5
- 12.Duran-Montgé P, Theil PK, Lauridsen C, Esteve-Garcia E. Dietary fat source affects metabolism of fatty acids in pigs as evaluated by altered expression of lipogenic genes in liver and adipose tissues. Animal. Published online 2009:535-542. doi:10.1017/s1751731108003686
- 13.Realini CE, Duran-Montgé P, Lizardo R, Gispert M, Oliver MA, Esteve-Garcia E. Effect of source of dietary fat on pig performance, carcass characteristics and carcass fat content, distribution and fatty acid composition. Meat Science. Published online August 2010:606-612. doi:10.1016/j.meatsci.2010.03.011
- 14.Duran-Montgé P, Realini CE, Barroeta AC, Lizardo RG, Esteve-Garcia E. De novo fatty acid synthesis and balance of fatty acids of pigs fed different fat sources. Livestock Science. Published online August 2010:157-164. doi:10.1016/j.livsci.2010.05.017
- 15.Huong DTT, Ide T. Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats. Br J Nutr. Published online July 2008:79-87. doi:10.1017/s0007114507876227

In the first study it’s interesting that the lipogenic enzyme activity was highest in the olive oil group yet they didn’t have the most weight gain. The weight gain itself seemed to track with the amount of linoleic acid. It seemed that olive oil increased liver triglycerides though. I’m going to have to look at this study closer to see why that might be.
One thing to know about oleic acid is that it produces oleoylethanolamide (OEA) when eaten. This OAE has a lot of effects, including that OEA is a PPAR alpha agonist. Activating PPAR alpha is “good” in the sense that it increases fat oxidation but “bad” in the sense that it floods the mitochondria with fat, which could potentially cause reductive stress, especially if that fat is MUFA. PPAR activators put mice into torpor.
FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption
FXR agonists are used to treat non-alcoholic fatty liver disease (NAFLD), in part because they reduce hepatic lipids. Here, we show that FXR activation with the FXR agonist GSK2324 controls hepatic lipids via reduced absorption and selective decreases in fatty acid synthesis. Using comprehensive lipidomic analyses, we show that FXR activation in mice or humans specifically reduces hepatic levels of mono- and polyunsaturated fatty acids (MUFA and PUFA). Decreases in MUFA are due to FXR-dependent repression of Scd1, Dgat2, and Lpin1 expression, which is independent of SHP and SREBP1c. FXR-dependent decreases in PUFAs are mediated by decreases in lipid absorption. Replenishing bile acids in the diet prevented decreased lipid absorption in GSK2324-treated mice, suggesting that FXR reduces absorption via decreased bile acids. We used tissue-specific FXR KO mice to show that hepatic FXR controls lipogenic genes, whereas intestinal FXR controls lipid absorption. Together, our studies establish two distinct pathways by which FXR regulates hepatic lipids.
https://pubmed.ncbi.nlm.nih.gov/34270928
The Italians have known this since ancient times: https://fireinabottle.net/campari-is-a-dual-bile-acid-receptor-agonist-that-activates-ampk/
Steve, I noticed that. mono vs poly?
I guess more fat must be created and then burned – less torpor?
Hi Brad,
I know this question doesn’t have much to do with this particular part of your blog but could I please get your opinion on Coenzyme Q 10??
I eat a fair bit of offal with beef heart quite often featuring on my plate!! I noticed that this is hugely loaded in Q10 and from what I could see it seems to be a good thing for metabolism but it is technically an anti oxidant!! So do you feel this would help or hinder me in my quest to achieve a giant metabolic rate???
CoQ10 isn’t really an “anti-oxidant”. I don’t even thing “anti-oxidants” are real things. Vitamin C is sometimes an anti-oxidant, sometimes an oxidant. It all just depends. https://www.pnas.org/doi/abs/10.1073/pnas.0806433105
CoQ10 IS really crucial in mediating the response of superoxide generation by SFA vs PUFA and MUFA. For this reason, I wouldn’r necessarily mess with it, unless you’re REALLY sure you’re low in it. This isn’t medical advice, but I’d focus on things that regenerate NAD+: alpha lipoic acid, nicotinamide riboside, sterculia oil, succinate, l-carnitine….
Brad, I am a recent reader of your blog so late to the party but glad I found your site. I like the direction, paradigm breaking, and thought provoking discussions concerning the health benefits of SFAs, MUFAs, and PUFAs. Been Type 2 diabetic since 2018 and been through the literal meat grinder and multiple dietary interventions trying to resolve what used to be termed a “progressive and untreatable disease.” Was curious from a macro economic and dietary level, have you read the semi recent article in Frontiers in Nutrition titled “United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-communicable Diseases.” 13 January 2022 There are a number of inescapable similarities between fundamental changes in American dietary practices and the reduction in SFAs and the proliferation of vegetable oils, HFCS and overprocessed and overengineered “food” products.
Hi Jeremy!
I haven’t read that particular study but its conclusions seem inescapable. I’ve yet to hear someone make a reasonable argument as to what makes saturated fat bad in the first place even though I’ve heard it my whole life. It’s clear that humans are a hunting species, we’ve always eaten saturated fat, why is it now bad? I mean, if anyone has evidence I’m happy to look at it, but I haven’t seen any.
Brad