After publication of The Croissant Diet, which promotes a diet combining highly saturated fat and starch for weight loss, I got a lot of questions along the lines of whether it mattered if fat was eaten before starch. Or maybe protein should be consumed first? The proposed mechanism of the croissant diet is that saturated fat causes fat cells to be insulin resistant which caused them to reject storing energy from blood glucose, fat, etc. Several questioned whether saturated fat could really be digested and transported quickly enough to shut down insulin signalling to fat cells before nutrient could be stored from the meal.
All of this enhanced the concerns I had voiced in “Introducing The Croissant Diet” about whether dietary fat was burned preferentially to stored fat. Is there a LIFO (Last In, First Out) mechanism for metabolism.
The LIFO Mechanism
Lambert and Parks 2012 review is a good place to start thinking about what happens to dietary fat after a meal. Their Figure 1 is as good of a summation of how dietary fat is treated as I’ve seen.
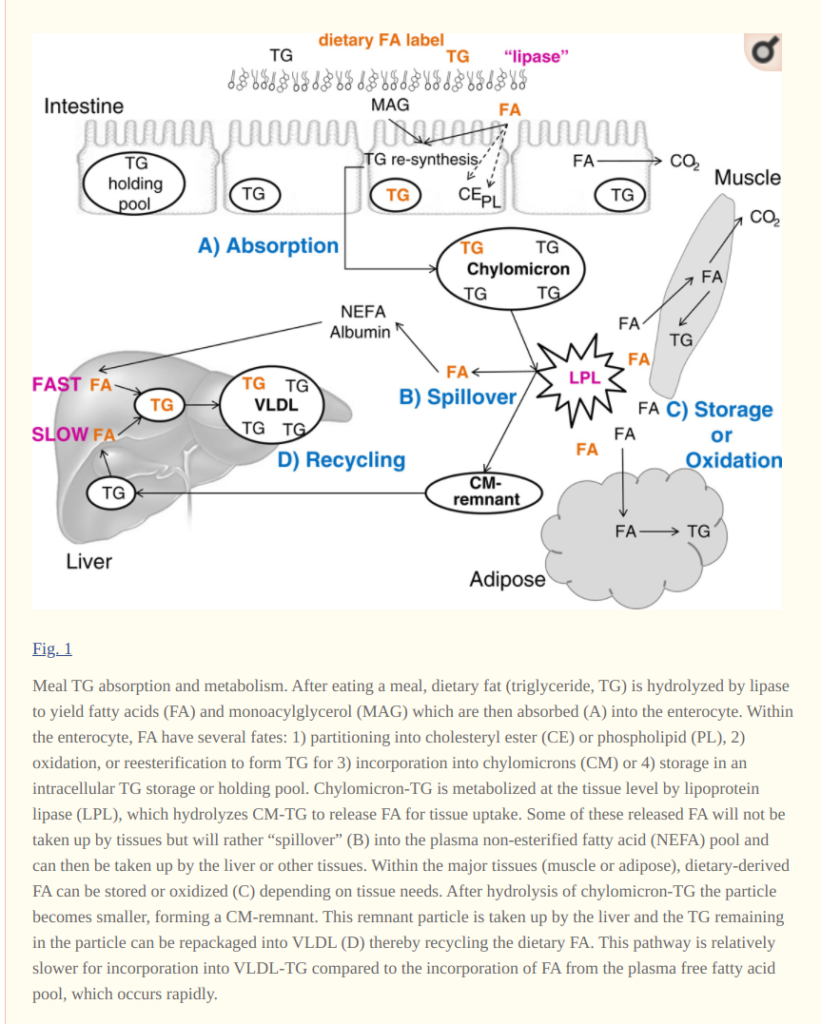
Fat, including dietary fat, is stored as “triglycerides” – three fat molecules attached to a glycerol molecule. Triglycerides can’t be transported across membranes. In your intestine, lipase enzymes cleave the fats from the glycerol backbone, separating them into “fatty acids” (FA) which can be moved into enterocytes through fatty acid transport proteins.
Enterocytes are the cells that line the small intestine. Once inside the enterocyte, the fatty acids have several potential fates including being burned (oxidized) for fuel. Most of them are “reesterified”, which simply means turned back into triglycerides, packed into large bundles called “Chlyomicrons” and released into the bloodstream. Fat cannot simply be released into the blood in any quantity because it is not water soluble. So the enterocytes convert a certain amount of the fat to phospholipids. Phospholipids are just fats with a phosphate group on one end. Remember the “phospholipid bilayer” from high school biology? That’s how cell membranes work. The fatty chains stick to each other in the middle along with fat soluble proteins. The phosphate groups move to the outside because they are water soluble.
So a chlyomicron is a blob of fat surrounded by a single layer of water soluble phosphate groups which allows it to happily float through the blood. This is the same way detergent works. The detergents have a fat soluble end that stick to fats and a water soluble end that make the fat blobs water soluble. Cool, huh? This is also the same way mayonnaise and butter work. This also explains the problem with term “artery-clogging saturated fat.” The saturated fat doesn’t float in the blood, it’s packed into a water soluble chlyomicron. Other fat soluble parts of food can be packed into chlyomicrons as well – cholesterol, Vitamins A, D, E and K, etc.
The chlyomicrons float in the bloodstream until they encounter an enzyme called lipoprotein lipase (LPL). The LPL is attached to the membrane of epithelial (capillary) cells in various tissues and it is the enzyme that cleaves the triglycerides back into fatty acids, which can then be taken up by the tissues. When chlyomicrons encounter LPL they “park” there for a while and unload their cargo as fatty acids. The tissues taking up the fatty acids have the option to store them away or burn them immediately.
When fat is oxidized in the mitochondria, it has to be first cleaved by a lipase enzyme. It is the fatty acid, as opposed to the triglyceride, that enters the mitochondria. It makes sense that cells taking up fatty acids would oxidize (some of) them immediately. But do they? The best way to tell is to feed someone fat that is radioactively labelled and see how quickly the fat is oxidized and released in the breath or urine. And indeed, dietary fat is already being oxidized in the body within two hours of being ingested. The following is a table from the same review article.
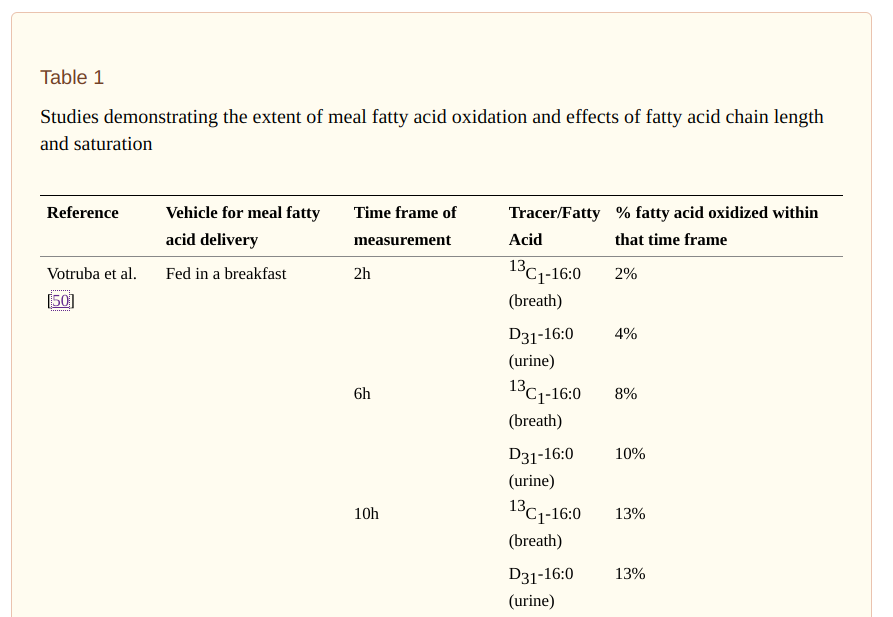
Within two hours of eating a mixed meal containing fat, 6% of that fat had been oxidized and the radiolabeled products released in either breath or urine. After 10 hours, 26% of ingested fat had been oxidized. Clearly, one does not oxidize 26% of overall body fat stores in a ten hour window, so the tracer studies show us that indeed there is a Last In First Out mechanism of preferentially burning dietary fat over stored fat as the nonesterified fatty acids enter the tissues.
Other things I learned from tracer studies: as much as 48% of ingested fat is oxidized in the first 24 hours and 98% of dietary fat is cleared from chlyomicrons in 24 hours.
The Triglyceride Holding Pool
If you look at the first figure you’ll see that the enterocyte on the left has a “TG Holding Pool”. What is that about? According to the authors:
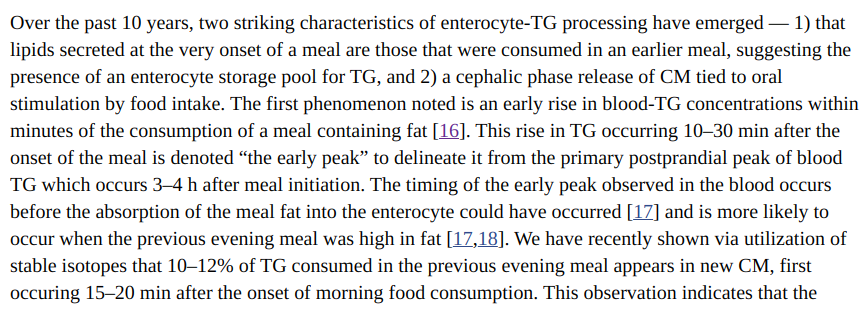
This is the answer to the question of how can dietary fat be absorbed quickly enough to disable insulin-stimulated influx of calories into fat cells. It can’t be, so the enterocytes hold a little bit back to prime the pump for the next meal. If this is the first meal of the day, this first released pool of chlyomicrons disappear from the bloodstream very quickly. Look at the charts of chlyomicron concentrations in individuals after tasting fat (the full paper):
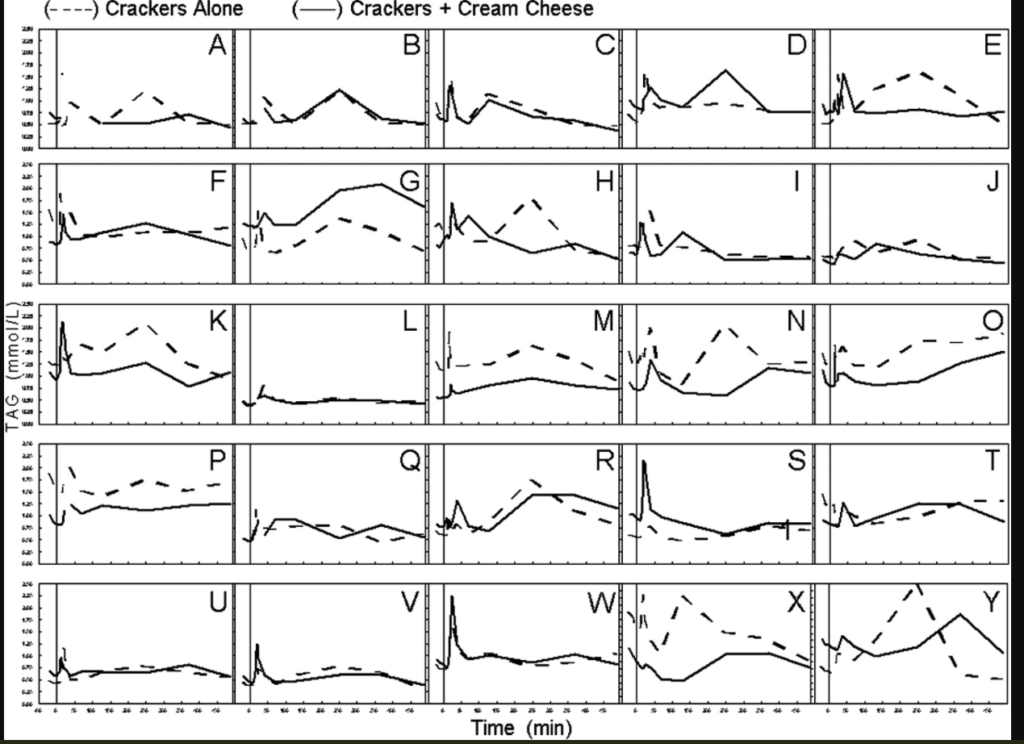
Within minutes of eating, blood levels of fat spike and then are cleared from the blood, presumably because they are taken up by tissues as fatty acids that will then preferentially burn them. This all happens before energy availability of the current meal becomes available as blood glucose or chlyomicrons. Where does the fat end up? This paper shows that chlyomicrons localize in tissues expressing LPL.
Which tissues express LPL? Fat tissues, skeletal muscle, the heart and the hypothalamus. LPL activity is highest in abdominal fat.
Putting It Together
In The ROS Theory Of Obesity I suggested that the mechanism for which long chain saturated fat produced mice with very little abdominal fat was by driving ROS production at the mitochondrial bottleneck which knocks out insulin signalling in adipose tissues and therefore prevents fat cells from switching from fat burning mode into glucose burning/fat storage mode. In my croissant diet experiment, the biggest change I noticed was a reduction of abdominal fat, where LPL activity is greatest.
I noticed during the experiment that the consumption of long chain fats created satiety, especially after the first days. Many others who have tried the diet have reported the same effect on satiety. Satiety is in part signaled by ROS generation in the hypothalamus, which also expresses LPL.
Conclusion
Within minutes of eating, your enterocytes release a quick burst of dietary fat from your previous meal in chlyomicrons. The fat in these chlyomicrons are rapidly taken up by tissues that express LPL and begin being oxidized immediately. Major sites of the oxidation of these fats include fat cells, especially abdominal fat, and the hypothalamus. This fat oxidation seemingly happens before the major blood sugar and insulin rise from the meal. If the fat consumed in the previous meal is long chain saturated fat, the initial fat burst will lead to decreased fat storage, especially in abdominal fat and increased satiation due to hypothalamic ROS production.

Aren’t those called “Chylomicrons”?
For some reason I can’t keep it straight. I really like the band Rilo Kiley. Chylo Rilo Chlyo Kiley. It’s like a mind blender.
love all your stuff and have been following with bated breath. So. What happens if you haven’t eaten for >24hrs?
It’s unclear. I think the longest tested break was 16 hrs. It seems to me like if the mechanism is to hold onto the fat until a meal is eaten, they probably hold on to the fat indefinitely. But that’s just speculation.
Only speculating, but if the enterocytes drew on that fuel themselves also, this could explain the research suggesting higher insulin sensitivity in the morning.
It seems to be well known that at some point during a prolonged fast triglycerides rise in blood. I did a lipid test after a 40 hrs fast & my Trigs were thru the roof. I’m not at all sure if this is directly related to Trigs being released for energy or what, but it does happen.
my recollection is that chylomicrons are being produced and released continuously. Sooner or later the fat that’s hanging around in there is going to
Leave
Chlyomicrons are produced in response to a meal. The liver constantly repackages Free Fatty Acids into VLDL, similar to chlyomicrons – collectively they are most of the “triglycerides” when you get a blood test.
Triglycerides are mostly chlyomicrons plus VLDL, which is made in the liver by repackaging Free Fatty Acids released from fat cells. When you fast your fat cells release more fat as fuel, which can end up in VLDL, which are “triglycerides”.
the hypothalamus seems not to be subject to inflammation with long chain FA’s. Hypothalamci inflation stimulates feeding.
How do you think, a coffee with heavy cream “greases the skids” in the am? I also eat 85%+ chocolate/cocoa butter prior to retiring. Wonder how that works for metabolism?
I had the same thoughts about morning coffee with cream. Chocolate before bed might prime you for satiation when you have your coffee. It’s a little unclear!
n=1 I eat 90% chocolate melted in some heavy cream in the evenings. After I have my morning coffee with heavy cream next meal can be 8-9h later.
Gabor would say that the enterocytes secrete chylos but also bound is LPS….and sampled by mesenteric lymph nodes. These CM’s go directly into the system circulation via thoracic duct. However, some must go through the portal system to liver , prior to processing and release into systemic circulation.
How much “Fire In A Bottle” do you need to eat per day per 1800 calorie diet. Is 2 tablespoons at breakfast and 1 tbls at lunch and 1 tbls at dinner enough?
I think that will depend on the individual. I find it satiating enough that I rarely have more than two meals if I’m using a lot of it and avoiding unsaturated fats. 4 tbsp per day is a pretty good dose for most, I’d think.
mitochondria get “jacked ” (showing markers of fat burning) within 3 hrs of C:18 ingested fat.
https://www.nature.com/articles/s41467-018-05614-6
Interesting how that article vilifies C16:0 palmitic acid but lauds C18:0 stearic acid. I wonder if there is really something to that or if palmitic wasn’t studied as closely as stearic and it’s just the anti-saturated-fats biases taking.
I suspect its the second.
Brilliant article, I am finding so many answers to my questions about why true true traditional Fr , CH, Italian , Monégasque cuisines were so healthy, the people so thin and food so delicious! Not to mention they are the longest lived, had/have the least metabolic diseases … It’s the Butter stupid! Brilliant!
I bought some “Food Grade” (NF USP) Stearic Acid from Amazon so I could get started. It says it’s derived from vegetable fat. Does that matter? Would that somehow be different than animal sourced SA? How much (in grams) would you say that you consume per day? Do you think it would have the same fat burning/muscle building effect on a Carnivore diet or is the starch an essential component?
The stearic acid should be the same from plants as from animals. It shouldn’t matter. 20 g is a good target, I think. The “banana shake study” used 24. The starch may not be necessary, although it does seem to help some lower fasting Blood Glucose (more on this in an upcoming post).
I couldn’t order your stearic acid butter. Please ship to Canada, too!
I do now!
Completely off topic, but can you recommend a French cookbook?
Yes, for the easiest out there, Simple By Chef Mallet! Modern, easy 4 ingredients and you can add stearic acid really easily… if you are a a gourmet cook, Brad will know best!
Rosemary suggested Tania Teschke’s …The Bordeaux Kitchen. I really like Elizabeth Luard’s “The Old World Kitchen”, lthough that’s not specific to France and I like The Physiology Of Taste by Brillat-Savarin, although that’s not really a cookbook… They’re both sort of “prose about food with recipes”, which is why I like them.
Thought provoking, and a mechanism I had never heard of. Would definitely help explain why PUFA makes abdominal fat more prevalent and why SAFA would instead lead to uptake of other tissues. Furthermore, it would also give an elegant explanation of why starch/sugar in relatively high amounts wouldn’t matter if your ratio of saturated to unsaturated fats is really high.
I’m LCHF most of the time. I hope (actually I know) the croissants aren’t necessary because I’m quite sensitive to gluten. I really do love croissants though, one of my favorite cheats. I know that there is going to be a price to pay later though.
The best French Cookbook ever is Tania Teschke’s …The Bordeaux Kitchen!!!!
I’ll have to look it up!
Brilliant. Just one question (and i do not want to contradict your explanation as the measurements show it´s right!): how to the Enterocytes know that you were eating recently so they have to release the stored fat? I mean your meal first hast to pass your stomach, which might take a while? Whats the mechanism here, is it some kind of GIP from k-Cell signaling?
I don’t know the exact mechanism, but it seems like they dump their cargo when the next meal is ingested. Therefore any stored fat would HAVE to be from the last meal. Someone did an experiment – I don’t have the link at the moment – where they fed volunteers a highly unsaturated fat, then they fasted, then they were fed a high sat fat meal, the chlyomicrons that hit the bloodstream were collected and were full of highly unsaturated fat. From the previous meal.
I’ve been unable to lose weight, and in spite of being nearly straight ZC carnivore (coffee and cream) was still gaining. Now I don’t eat until I feel my blood sugar start to tank (and will provoke a migraine) – I go work out to release glycogen, and then I do coffee with your ghee-stearic acid production. In a week I’ve lost 3 pounds and it seems to be coming from belly fat – more to go, but thanks for your hard work – I have a little hope I can manage the turn around I’m seeking now.
Excellent. Good luck!
Hi Brad, thank you for this explanation. I started using your butter spread yesterday and the serving size is a tsp. So for 4 Tbsps daily that would be 12 servings a day. I’m gonna need to reorder pretty damn quickly LOL! And for clarity: eat this fat at each meal for full effect? And at the beginning or end of the meal doesn’t matter- or it depends on what the meal consists of? (Sorry not great at science).
I think the eating pattern depends on what works for you. In my case, the butteroil seems to help mo to go long periods with feeling much hunger. But I eat a lot at a time – two tbsp or more. But I’ve only been eating one large meal (feast) per day.
Hey Brad. I’m 40, female, stable weight around 54kg 167cm. I’ve been doing 5:2 since 8 months to support my husband who was even though looking slim fit, had pre-diabetic level HbA1C. And it worked we decreased it to normal level within 5 months so now doing sort of 1day/wk fast only (600kcal). We do Mediterranean diet, avoiding all refined flours, only sourdough whole-grain bread. Then I read your blog introducing the Croissant Diet on Jan 15th and the idea to eat croissants regularly hits me:) I’m Turkish, we have great butter, but for years have been trying to limit it to breakfast only and lunch&dinner would be with olive oil (also pushed my parents to change their ways to mine-feel very bad now:)), and some occasional flax oil because it is promoted a lot lately by anti-aging gang here, and of course nuts, daily and a lot. So I increase my butter, milk cream, cheese, decrease nuts significantly, decrease even olive oil, stop flaxseeds, stop whole grains, decrease protein, add croissants and white bread (I’ve even fitted a croissant containing meal to my fast day). I follow 42%carb 18%protein 40%fat macros if it is a croissant day for me. This was an experiment for 10 days because I know I start gaining weight immediately in 4-5 days if I do some stupid diet intervention:) There is no weight gain, I lost 1cm of my waist (I thought My waist was at its slimmest:)), and same with my husband, he even lost a little weight, though we don’t want that. No muscle loss so far, with less protein. I will definitely continue. This is a very happy ending for me after all those years trying to avoid “artery clogging saturated fat” 🙂 I eat my butter. And bread!
Yayyy!!! Happy day! Thanks for sharing!
This makes a lot of sense. Years ago when “The Biggest Loser” was a popular TV show, and we had such things as TV shows… I was reading the Merck manual on exercise energy production, because the point of the show was that 30 minutes of exercise was a waste of time since it was just enough to switch you to “fat burning” and not enough to actually burn any fat.
I went back to read your ROS article. I remember the H+ proton image in that manual from back then. Though I admit Hyperlipid is sometimes beyond my kenning. He’s definitely talking about something real though.
What you’re saying is offering the first explanation for why doctors always say “It must be genetic” when they see my cholesterol numbers. Aren’t they cute? They keep insisting on doing those tests. I keep telling them I eat mostly keto. So when LDL is high and TG is low, I’m doing well. When the opposite, I’m eating too much carb.
So the theory I”m developing, based on what you’ve said is, I’m eating the wrong fats, so it’s locking the TG out of the cells whenever I eat too much carb.
Or am I wrong there? I wish I could ask a doctor, but they’re just not down with this yet. Mine usually pulls the “Well, there are many things we don’t know about the body” line when I ask too many questions.
At least he’s honest.
heyyy my question to you is why do you wanna burn fat ? and why are you doing the keto diet? Carbs sugar and saturated fat….this combination is key!!
Ha! I would be SHOCKED if your doctor knew anything about this! But yes, the idea is that unsaturated fats plus carbs are stored very efficiently as body fat but carbs with highly saturated fats are not.
Hi Brad, maybe you didn’t like my previous comment or it didn’t post. Can I ask, if I received the butter flavored SA, is the full pound of stearic acid still coming? And you think it will take tablespoons daily to lose weight – and not teaspoons which is how the serving sizes are listed on the butter spread?
I am not complaining, just wanting to make sure I am doing this right and very happy to support your endeavors. Thank you.
Hey! Sorry, I haven’t had as much time to keep up with comments as I’d like but I’m catching up now. If you didn’t get something in your shipment can you email me (brad at fireinabottle dot net)? The serving size is just the industry standard for ghee. I would try to eat at least two tablespoons.
It must also be said that short and medium fats do not require activity from bile unlike long chain ones. Can this lead to differences?
I think it would be REALLY GREAT if someone could create a high quality dark chocolate made with stearic acid! I think ordinary chocolate is a source of unsaturated and hydrogenated (?) vegetable oils.I’m gonna melt some pure cacao with stearic acid and honey and see what happens!
Actually, most good chocolate is VERY high in stearic acid. It’s a great source! Cheap chocolate “things” are often made with cheaper palm oil. For instance, I was looking at Hershey’s Chocolate Syrup in the store the other day – palm oil. But if it says cocoa butter you’re good to go!
good stuff and a late happy new year to ya! How are you doing are u really lean now?? how is your journey going? all the best from nyc!
Chris rocco
Happy New Year! No, I just ate regular holiday food and gained some of the wait back. I started the diet retest Thursday, Jan 30th. I’ve had steak and pasta with butteroil, chilaquiles, fried chicken with mac-n-cheese, chocolate chip cookies for deserts and I’m already down 6.6 lbs in 6 days, so I’m doing pretty great! This time I’m going to stay on the diet indefinitely and see what happens. I’m doing one big meal in the evening. It works, not much hunger!
This makes me bring up my question of wether we can fat load as opposed to carb loading for sustained energy during long term cardio type exercise. ie. On feet, hiking all day.
Also, if there is an immediate release of fat upon eating fat that then either gets stored in tissues or immediately oxidized, would it make sense to sip long chain fatty acids throughout the day to potentially help continuously tap into the cycle of oxidizing body fat for energy?
I can tell you that if I’ve had too many carbs and begin to come out of ketosis during long hikes, I will feel my legs start to burn. Depleted glycogen I assume. However, a few sips of fat nearly immediately give me my legs back and the burning sensation is gone. I can continue all day as long as I sip fat.
Seemingly a few sips of fat would immediately release the stored fat as chlyomicrons, so there’s some sense in that. As long as the fat is saturated enough, it should stay in the bloodstream as Chlyomicrons/FFA to give you all day energy.
I have to confess I was initially very skeptical of your approach. But then I read more of your posts to understand the reasoning behind ROS-induced insulin resistance and leanness, in addition to Hyperlipid’s recent commentary on your work.
I do cyclic keto with carb backloading in the night. So I started to finish my evenings with more starch + butter as dessert (or something similar). Replacing nuts with alternatives containing more saturated fats (like unsweetened chocolate). More satiety, better mood, better sleep. And, as far as I can tell, I’m gaining lean mass but keeping a flat belly (keeping visceral fat low has been a primary concern of mine). Will have to verify with a DEXA in the future.
Anyway, it’s like getting permission to have fun again. Thanks for your writing.
Awesome! Thanks for reporting back. If we don’t have fun what do we have?
Are you still planning to ship the stearic acid orders soon/
They are shipping now!
Thanks, Brad! Just to confirm: the 2-4T suggested daily intake refers to the (20%/80%) SA/butter mix… not to the SA component alone, correct?
In reading the answers given to the ‘daily dosage’ questions, I got a bit confused on that point even though seems obvious that 2-4T MUST be the SAbutter measure, not the SA…. Started imagining having to eat a half pound of butter to obtain 45-60g of SA per day, whoa! 😀
Well, if you’re target is 60g, that is actually quite a lot of butter! The banana shake study suggests that 24g is plenty.
http://ota.bln.mybluehost.me/fiab/this-is-what-fat-burning-looks-like/
Hi Brad, I was wondering if you had any thoughts on implementing this on a relatively low-fat diet?
I’ve done high-fat diets and run Keto 3 time but it just doesn’t work for me.
I note that the mice were on a 40% fat diet which isn’t exactly low fat but a long way off low carb or keto.
My macros look like 50:30:20 Carb:Protein:Fat. I eat a lot of fruit and vegetables, a lot of fairly lean meat, potatoes and sweet potatoes, and a bit of white rice.
Obviously there is nothing to stop me making that 20% fat predominantly saturated but any thoughts on wider dynamics and how it might work on a diet like this?
I don’t have any real experience with this on a low fat diet… But the principles would be the same. Eliminate PUFA wherever possible and replace with SFA. Let me know how it goes, I’m curious!
Hi Brad,
Life is funny – saw a mention of your site last week and since then been reading constantly and placed not one, but 2 orders within a few days
Question for clarification
I’m keto carnivore (primarly, cough cough) and want to try my current WOE with the addition of the SA infused butter oil (receiving today hopefully) and/or supplemented SA
here is where I am confused – I have read that some people gain weight on a beef heavy carnivore diet – in this case, wouldn’t all the stearic acid they are consuming lead to a weight balance or loss of weight. Personally, I am stuck and would like to further reduce, but my introducing prolonged fasting leads to loss of too much sleep and binge eating… my round about question I think is – if I am eating carnivore (specifically beef – either raw or cooked in butter/tallow) as a baseline, would the extra SA buffer fat gain and/or does the extra SA somehow a net fat burn
if you already aren’t roling your eyes at my question – it boils down to me figuring how a pound of steak + 2-3 tbs of butter oil is more slimming than a pound of steak
an excited new customer
Jay
Hey Jay!
Beef fat from most cuts like steaks, etc is around 45% saturated and about 15% stearic acid. The butteroil is around 70% saturated and about 27% stearic acid, so it will actually up your ratios quite a bit by adding it to beef.