Video Link First, tl;dw Summary Article Below
Summary
Omega 3 PUFA activate PPAR alpha, which in turn activates peroxisomes. This is what gives PPAR alpha the name Peroxisome Proliferator.
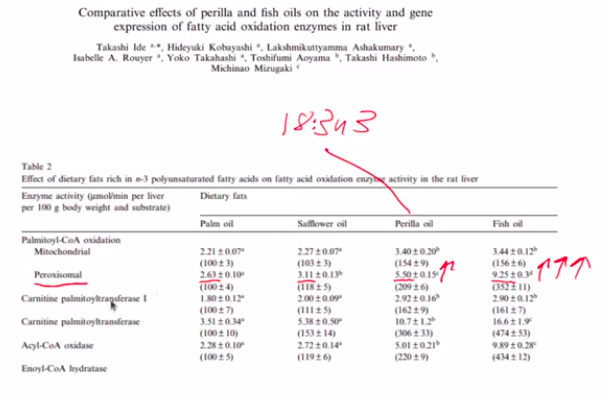
Peroxisomes carry out the process of beta-oxidation: breaking down long fat molecules into acetyl-CoA. Acetyl groups are 2 carbon units that are the building blocks of longer fats.
Peroxisomes can’t turn the acetyl-CoA into ATP – they don’t have an electron transport chain – so they export it. The exported acetyl-CoA can either be imported into the mitochondria to make ATP or it can be used in the cytoplasm to make fats, cholesterol and bile acids.
When PPAR alpha is activated, the result is an increase in both fat oxidation – fats being broken down into acetyl-CoA in the peroxisome and mitochondria – and lipogenesis – the rebuilding of the fats into mostly MUFA. You can see that fenofibrate – a pharmeceutical activator of PPAR alpha – increases expression of fat oxidation enzymes such as Aox (peroxisomal) and CPT-1a (mitochondrial). It also increases expression levels of enzymes involved in fat synthesis such as fatty acid synthase (Fas) and enzymes involved in creating MUFA such as Elovl6 and SCD1.
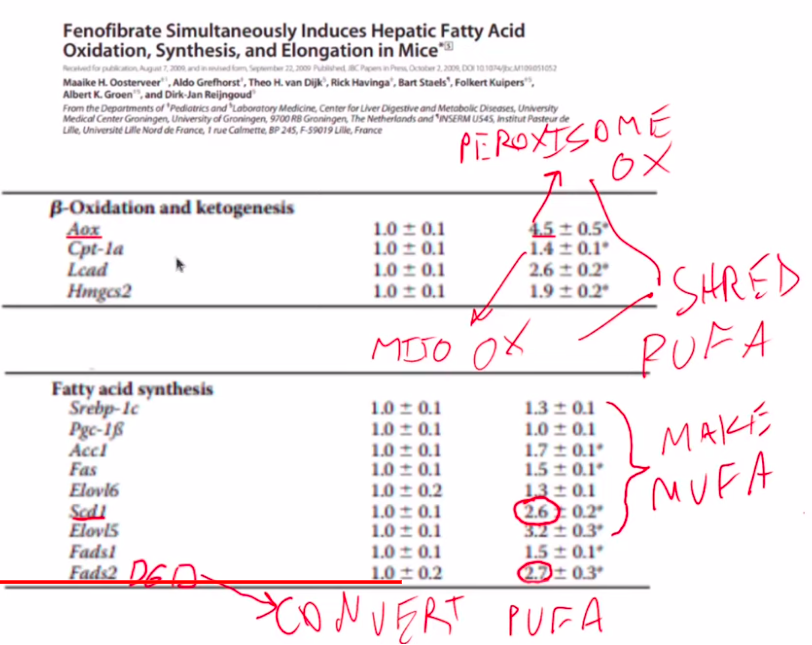
Activating PPAR alpha depletes PUFA and Increases Oleic acid
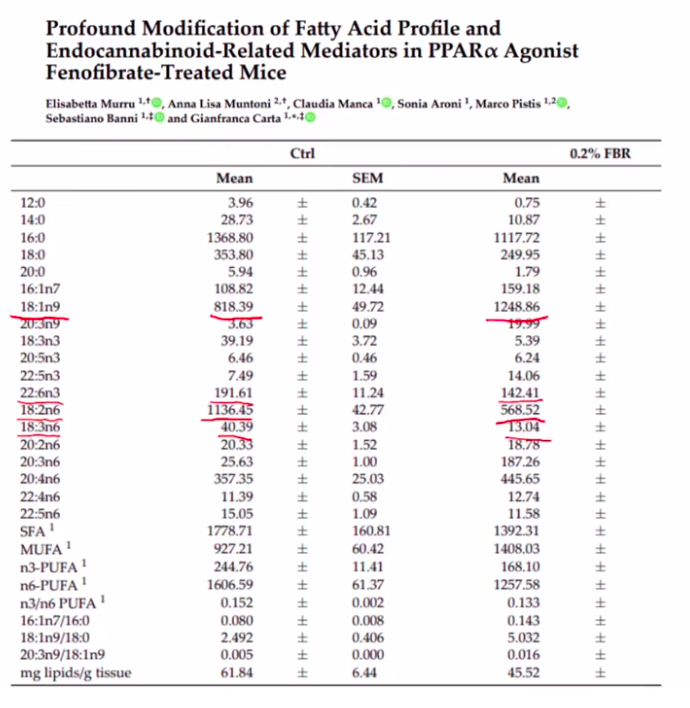
Getting rid of PUFA might sound great. In fact increased expression of the enzymes SCD1 and D6D are associated with massively increased risk of developing diabetes. (Kroger, 2011)
Fish Oil Kills Mice Lacking PPAR alpha
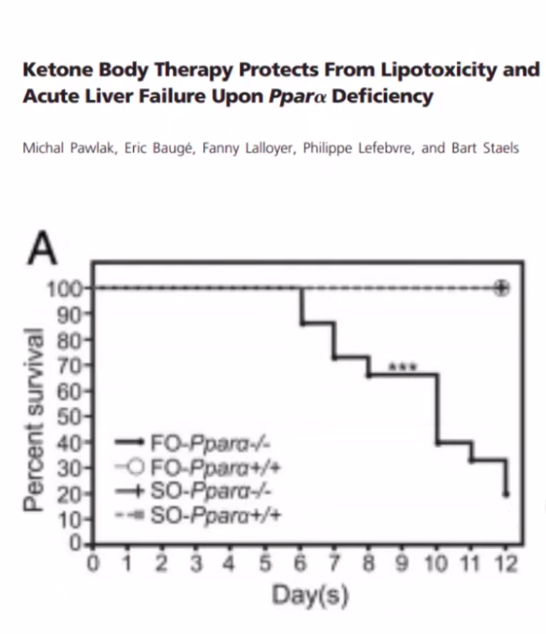
In addition to being involved in fat metabolism, PPAR alpha is also involved in detoxification pathways. If you remove the gene PPAR alpha from mice and feed them sufficient fish oil, they will be dead within two weeks due to acute liver failure.
Humans Have Less Peroxisome Activity Than Mice
Rodents eat PUFA-rich seeds and have high peroxisome activity. Humans evolved from fruit eating great apes who would’ve consumed little PUFA. Humans have lower baseline peroxisomal activity than rodents (and less ability to detoxify omega-3 PUFA) and human peroxisomal activity is increased less by PPAR alpha.
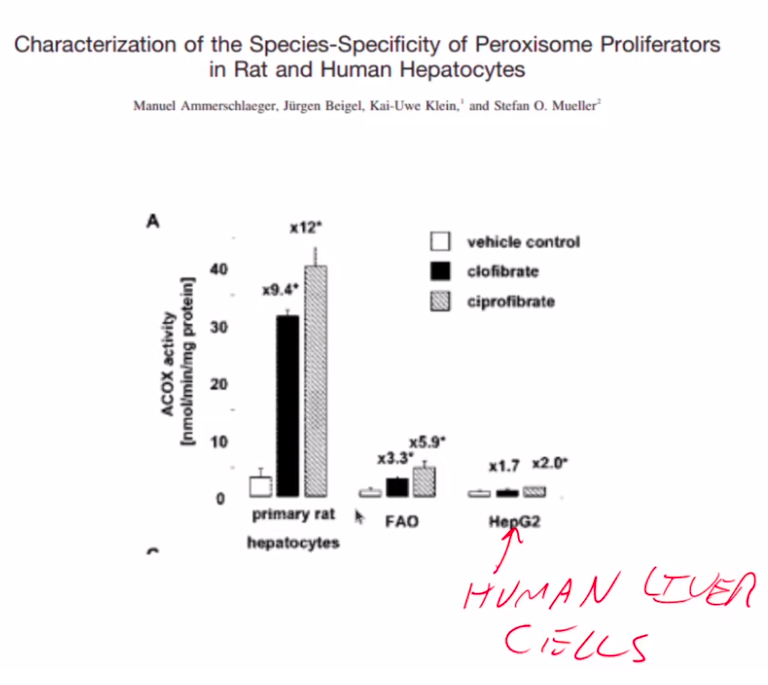
Maybe Don’t Eat PUFA?
Just a thought.
Ammerschlaeger, M. (2004). Characterization of the Species-Specificity of Peroxisome Proliferators in Rat and Human Hepatocytes. In Toxicological Sciences (Vol. 78, Issue 2, pp. 229–240). Oxford University Press (OUP). https://doi.org/10.1093/toxsci/kfh071
Burdge, G. C. (2006). Metabolism of α-linolenic acid in humans. In Prostaglandins, Leukotrienes and Essential Fatty Acids (Vol. 75, Issue 3, pp. 161–168). Elsevier BV. https://doi.org/10.1016/j.plefa.2006.05.013
Claudel, T., Cretenet, G., Saumet, A., & Gachon, F. (2007). Crosstalk between xenobiotics metabolism and circadian clock. In FEBS Letters (Vol. 581, Issue 19, pp. 3626–3633). Wiley. https://doi.org/10.1016/j.febslet.2007.04.009
Hansen, R. P., & Czochanska, Z. (1975). The fatty acid composition of the lipids of earthworms. In Journal of the Science of Food and Agriculture (Vol. 26, Issue 7, pp. 961–971). Wiley. https://doi.org/10.1002/jsfa.2740260713
Ide, T., Kobayashi, H., Ashakumary, L., Rouyer, I. A., Takahashi, Y., Aoyama, T., Hashimoto, T., & Mizugaki, M. (2000). Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. In Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids (Vol. 1485, Issue 1, pp. 23–35). Elsevier BV. https://doi.org/10.1016/s1388-1981(00)00026-3
Käkelä, R., & Hyvärinen, H. (1995). Fatty acids in the triglycerides and phospholipids of the common shrew (Sorex araneus) and the water shrew (Neomys fodiens). In Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology (Vol. 112, Issue 1, pp. 71–81). Elsevier BV. https://doi.org/10.1016/0305-0491(95)00058-g
Kröger, J., Zietemann, V., Enzenbach, C., Weikert, C., Jansen, E. H., Döring, F., Joost, H.-G., Boeing, H., & Schulze, M. B. (2011). Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. In The American Journal of Clinical Nutrition (Vol. 93, Issue 1, pp. 127–142). Elsevier BV. https://doi.org/10.3945/ajcn.110.005447
Murru, E., Muntoni, A. L., Manca, C., Aroni, S., Pistis, M., Banni, S., & Carta, G. (2022). Profound Modification of Fatty Acid Profile and Endocannabinoid-Related Mediators in PPARα Agonist Fenofibrate-Treated Mice. In International Journal of Molecular Sciences (Vol. 24, Issue 1, p. 709). MDPI AG. https://doi.org/10.3390/ijms24010709
Oliver, M. (2012). The clofibrate saga: a retrospective commentary. In British Journal of Clinical Pharmacology (Vol. 74, Issue 6, pp. 907–910). Wiley. https://doi.org/10.1111/j.1365-2125.2012.04282.x
Oosterveer, M. H., Grefhorst, A., van Dijk, T. H., Havinga, R., Staels, B., Kuipers, F., Groen, A. K., & Reijngoud, D.-J. (2009). Fenofibrate Simultaneously Induces Hepatic Fatty Acid Oxidation, Synthesis, and Elongation in Mice. In Journal of Biological Chemistry (Vol. 284, Issue 49, pp. 34036–34044). Elsevier BV. https://doi.org/10.1074/jbc.m109.051052
Pawlak, M., Baugé, E., Lalloyer, F., Lefebvre, P., & Staels, B. (2015). Ketone Body Therapy Protects From Lipotoxicity and Acute Liver Failure Upon Pparα Deficiency. In Molecular Endocrinology (Vol. 29, Issue 8, pp. 1134–1143). The Endocrine Society. https://doi.org/10.1210/me.2014-1383
Sharma, P., & Agnihotri, N. (2020). Fish oil and corn oil induced differential effect on beiging of visceral and subcutaneous white adipose tissue in high-fat-diet-induced obesity. In The Journal of Nutritional Biochemistry (Vol. 84, p. 108458). Elsevier BV. https://doi.org/10.1016/j.jnutbio.2020.108458
Singh, H., Beckman, K., & Poulos, A. (1994). Peroxisomal beta-oxidation of branched chain fatty acids in rat liver. Evidence that carnitine palmitoyltransferase I prevents transport of branched chain fatty acids into mitochondria. In Journal of Biological Chemistry (Vol. 269, Issue 13, pp. 9514–9520). Elsevier BV. https://doi.org/10.1016/s0021-9258(17)36911-9
Vessby, B., Lithell, H., Gustafsson, I.-B., & Boberg, J. (1980). Changes in the fatty acid composition of the plasma lipid esters during lipid-lowering treatment with diet, clofibrate and niceritrol Reduction of the proportion of linoleate by clofibrate but not by niceritrol. In Atherosclerosis (Vol. 35, Issue 1, pp. 51–65). Elsevier BV. https://doi.org/10.1016/0021-9150(80)90027-1

Great work, very interesting, thank you. Only one comment about your statement of lowering NADH by activating peroxisomes. Literature says the opposite is true in mouse model. It is part of the fat making setup, malonyl-CoA prevents saturated fats to go to mitochondria, so they are converted to unsaturated by SCD1 or wait, and high NADH prevent pyruvate to be oxidized in mitochondria, it is converted to glycerol to make fat droplets from triglycerides.
It looks like this detox mechanism of converting PUFA to oleic acid prolongs life of worms by lowering oxidative stress. PUFA with double bonds on even positions possibly harm NNT anti-oxidation chain by using DECR enzyme that depletes NADPH in mitochondrial matrix. And peroxisomal H2O2 is mainly used to lower lipolysis, fat making is protective and safe if NNT system is not harmed by acetylation.