It’s rare to find a scientific paper whose RESULTS are hilarious. Sometimes the authors conclusions are hilarious. If you’ve lived through the last 30 years of articles about the best sources of antioxidants and how to avoid oxidative stress, though, the actual findings of this study1 will certainly seem ironic, if not hilarious.
I’ve written about Nrf2 before. It’s a critical transcription factor in controlling antioxidant defense. For instance, when Nrf2 is activated by Reactive Oxygen Species (ROS), it upregulates the production of glutathione, the body’s “master antioxidant”. This is the body’s first line of defense against oxidative stress.
But what happens if you remove the Nrf2 gene from a mouse?
- Cellular buildup of hydrogen peroxide, AKA “Oxidative Stress”
- A large increase in Ucp1, AKA Mitochondrial Uncoupling
- Increased Metabolic rate
- Resistance to Obesity
- Improved glycemic control
- Lowered fasting insulin
Come on….. It’s funny!!
But of course the mice were suffering in all sorts of ways due to the massive oxidative stress. Right?!
According to the authors, “Mice lacking the Nrf2 gene demonstrate no obvious outward defects”.
They do seem to have some long term bad effects, including increased susceptibility to certain toxins and carcinogens. But mostly, they are normal mice with a souped up metabolic rate.
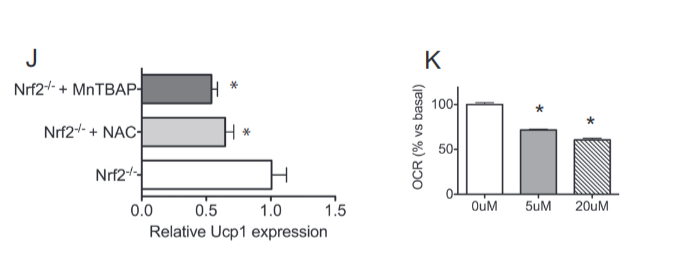
What happens if we culture some cells from these mice? Normal cell culture but with much higher oxygen consumption, AKA higher metabolic rate. And if we add some powerful antioxidants to the tissue culture? The oxygen consumption, AKA metabolic rate, drops.
Bro, My Mind Is Blown, Everything Is Backwards, How Can I Go On?
It’s going to be OK.
The body often has redundant ways of coping with problems. Glutathione is the first line of defense against oxidative stress. But Ucp1 is the second line of defense. The production of ROS is an inevitable result of oxidative phosphorylation, which is the broad term for how the mitochondria uses oxygen to convert the chemical energy in a fat or carbohydrate to ATP.
The mitochondria is like a battery. The electron transport chain pumps protons to the outside of the inner mitochondrial membrane, meaning that the area outside of the mitochondria has a positive charge and the area inside of the mitochondria has a negative charge, just like the two terminals of a battery. The mitochondria uses this voltage difference to do work. Complex V uses this voltage difference to convert ADP to ATP. ATP is the bodies primary fuel and is used to move muscles, for instance.
This process creates ROS. Normally, the ROS would activate Nrf2, which will increase glutathione, which will mop up the ROS. But if Nrf2 doesn’t exist, the ROS just build up as hydrogen peroxide. The cells response is to increase UCP1. UCP1 “uncouples” the mitochondria, meaning that it allows the protons to “leak” back through the inner mitochondrial membrane. This releases energy as heat, as opposed to using energy to do “work”: moving a muscle.
I introduced UCP1 in my last post.
The ability to simply let the protons flow back through the membrane reduces the voltage differential across the inner mitochondrial membrane. When the voltage differential is low, it’s easier to pump protons across the membrane by the enzymes in the electron transport chain. When the voltage differential is low, far less ROS are produced. The cell is saved from massive oxidative stress. It IS under oxidative stress, but it’s not that bad. Maybe there’s twice as much hydrogen peroxide around, but hydrogen peroxide isn’t that reactive. The cell can deal with it.
Inefficient Metabolism
The difference between the two scenarios – ROS being dealt with by glutathione vs. ROS being dealt with by UCP1 – is that with glutathione, metabolism is very efficient. Very little chemical energy is “wasted” as heat.
When UCP1 levels are high, the metabolism becomes very inefficient. You are losing energy that could be used to move muscles by just burning it off as heat.
Evolutionarily, inefficient metabolism is a BAD idea and would be selected against. Why would you want to waste hard earned calories as heat?! If you’re a modern fat person, the tables are turned. Maybe you want to be inefficient.
A surge of ROS in your mitochondria will get you there.
- 1.Schneider K, Valdez J, Nguyen J, et al. Increased Energy Expenditure, Ucp1 Expression, and Resistance to Diet-induced Obesity in Mice Lacking Nuclear Factor-Erythroid-2-related Transcription Factor-2 (Nrf2). Journal of Biological Chemistry. Published online April 2016:7754-7766. doi:10.1074/jbc.m115.673756

Great post, Brad! Evolutionarily, calories as heat would be critical in winter. Interestingly, under natural conditions, very little exogenous antioxidants would be available in winter.
What about all the SCD1 regulators that are also antioxidants? Is it worth it to inhibits SCD1 at the expense of lowered UCP1?
I don’t think of most of them as being classic antioxidants. Let’s take berberine, for instance. By lowering SCD1 activity, berberine drives MORE ROS production, yet it is said to have anti-oxidant properties. How do we reconcile these two facts?
UCP1 IS an anti-oxidant. By lowering the mitochondrial membrane potential, electrons flow more easily through the electron transport chain, creating less ROS. So a burst of ROS leads to higher metabolic rate and less ROS later.
Brad
If you consume Glucose and Stearic Acid together, does the cell prioritize fatty acid metabolize over glucose metabolism (physiological IR)? Wondering if there is a hierarchy change in the presence of stearic acid, whereas generally I’ve read that in the presence of insulin, glucose metabolism is favored.
PS: what happens to the insulin and glucose if fatty acid metabolism continues? Does the muscle take up glucose at a higher rate while the fat cells keep doing beta oxidation? Thanks!
Every cell/tissue will make a determination if it wants to use glucose or fat. This is determined by the Randle cycle and cells typically don’t burn both at the same time. My guess is that with high dietary SatFat, the glucose is shunted more into glycogen rather than going into muscle or adipose, but that’s not really clear at this point.
Brad
I use NAC to help with my cognitive function (focus, working memory, processing speed). I was suspected to have ADD as a child and I think this might be why I find it so helpful. Sadly, it looks like I’m going to need to choose between cognitive function and bodyweight. Do you think I could cycle the NAC to get the cognitive benefits on workdays and the ROS benefits on weekends, or do you think it hangs around?
Interesting. I didn’t realize NAC was a treatment for cognitive function. I don’t know what the half life of NAC is, but in general I like the idea of cycling things. If you did like four days on, three days off, I’m guessing you’d get a pretty big increase in ROS response in the three days off but IDK!
The half-life is something like six hours or so. I use the stuff as a mucolyticum and take 600mg thrice daily because it gets flushed out so rapidly
I’ve been using your stearic acid for a couple of weeks now with nothing to report. I see a hematologist next week for iron overload and will get weighed there. I can definitely tell the SA is there as it creates a slippery, slimy texture in my sauces and protein shakes.
It also leaves a greasy residue in my pots, pans and pressure cooker pot. When I wash it the dish detergent doesn’t seem to cut the grease like it should. Any recommendations?
Cast iron, my friend…you want that grease coating on cast iron so the stearic acid works in your favor here. Also setting cast iron on wood cutting boards, having a big wood bowl to break up my suet and store it, wooden cooking AND eating utensils(I use chopsticks a lot)…. Wood is a bit similar to cast iron in a way where it’s good to be greased up. No need for mineral oil(which you’re expected to use on wood) when you have highly stable saturated grease.
I’ve gone through 2 bags of his SA and started on my third. I was mixing it in my coffee with limited results. I found if I added some butter or mct oil it would bind with that and stay dissolved. But it was a pain. Lately, I’ve been mixing a heaping tablespoon into some Fage 5% plain yogurt with a small pinch of 100% stevia and cocoa powder. Pretty tasty.
Here’s my breakthrough though. I bought some 00 gelatin capsules and a loading “machine” and just fill the capsules up with SA. Now I can take it with all foods without having to worry about mix in. I can even take it when I’m fasting with no problems.
If I had to do it over again I’d have gotten the larger 000 capsules since I don’t have issues with large pills.
I take 6 with each meal.
Interesting. You are seeing results from this? I have concerns about absorption of the straight SA, but you’re not the first to express positive results.
Brad
Yes, the grease is a problem. Here’s an idea:
Commercial Degreaser
Good luck! Consider testing your Desaturase Index.
Hi!
Remember a study on moderate and heavy drinkers in Italy, long time ago, slim people 140 pounds (60kg). Those who consumed more than 1000 kcal of alcohol daily, had a TEE of 300 kcal higher. This is substantial.
Heavy drinkers open up a side chain of alcohol processing, MEOS aka microsomal way. The cytochrome p450/ CYP2E1 gene expression speeds up and creates more tolerance for alcohol, with a price: more ROS of all kinds. (Same gene nullifies paracetamol and warfarin, creating toxic byproducts).
I guess GSH is massively consumed in this sped-up process. Could it be that UCP1 comes to help with increased thermogenesis worth 300 kcal /d? The alcoholics had same or even better metabolic values than their comparison group, apart from liver ALT’s etc. Presume that wine (dry) was their staple; it is by far the cheapest alcohol available.
JR
Uh…greater susceptibility to toxins and carcinogens? That kinda seems like a big deal. Should someone who walks to work in a high traffic city consider antioxidants?
Well, I assume you have a functional Nrf2 gene, so no, I don’t recommend antioxidants.
A wild speculation…..perhaps ucp1 and uncoupling evolved with creartures that came live in environments away from underwater vents. Does not explain current conservation of pathway although more than one benefit is possible