In this series I’ve been using the analogy of a flooded engine to help to understand some pretty complicated dynamics that happen in the mitochondria when we burn fat. The details can be overwhelming but the high-level perspective is pretty straightforward. If you fill the mitochondria with too much fuel and not enough oxygen, the fuel won’t burn. Acetyl-CoA and NADH will build up, resulting in a flooded engine. The only way for the mitochondria to clear itself of the extra fuel is to turn on De Novo Lipogenesis (fat making). DNL packs the acetyl groups into the saturated fat palmitic acid and the monounsaturated fat oleic acid. The fat generated can be stored away as fat.
Carbon Recycling of PUFA
When you see fet being generated de novo, it is a sign that mitochondria are in reductive stress. When you eat plant based PUFA – linoleic acid (AKA linoleate) and a-linolenic acid (AKA a-linolenate), your body switches on DNL and rebuilds them into palmitic acid and oleic acid.1

When you consume these fats they are rapibly broken down to acetyl-CoA by beta-oxidation and then built right back into saturated fat (palmitic acid) and monounsaturated fat (oleic acid).
This phenomenon is called “carbon recycling”:1


“Why are LA and ALA extensively ‘carbon recycled’ into lipid synthesis?” Another way to ask this is, “why does the body tear down PUFA and rebuild it into saturated fat?” If you’ve been following along with this series, I’ll give you some hints and you can put the rest together.
- PUFA are natural activators of PPAR alpha, which upregulates CPT1, the enzyme that shuttles fat into the mitochondria.2
- PUFA are preferred by CPT1, the enzyme that is upregulated by PPAR alpha and that shuttles fat into the mitochondria.3
- PUFA have low activity at complex II and therefore fail to increase oxygen consumption when burned.
The first two facts mean that PUFA is substantially preferentially oxidized compared to saturated fat. This is easy to demonstrate in humans.4
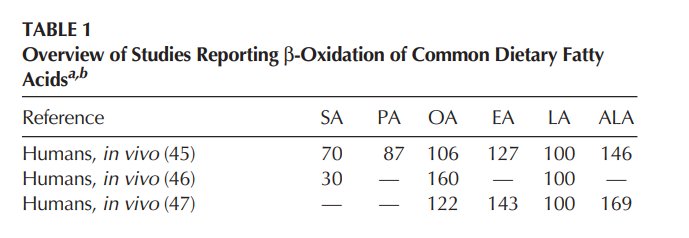
The organism controls the rate at which energy from fat enters the mitochondria primarily via PPAR alpha2 and CPT1. It controls how much oxygen it burns via the superoxide-glutathione reductase-NNT pathway. If you feed the organism a lot of fat which artificially stimulates the rate at which fuel enters while limiting oxygen burn rate and therefore fuel burned, you will certainly end up with a buildup of acetyl-CoA and NADH in the mitochondria. This is called reductive stress and the outcome is certain: De Novo Lipogenesis (DNL) and therefore the carbon being recycled into palmitate and oleic acid. From the perspective of the mitochondria, DNL is a defensive move. It’s a way to offload the extra acetyl groups and store them as fat.
The palmitic and oleic acids that are generated from PUFA is a predictable result. The PUFA basically overrides the cell’s control mechanisms, forcing the reductive stress and the DNL5. This is a really nice simple demonstration of some complex dynamics. If the rate of fat entering the mitochondria is artificially accelerated by PUFA and no extra oxygen is injected, the result is a buildup of acetyl-CoA and De Novo Lipogenesis. What else would you expect?
This absolutely happens in humans when they eat PUFA.6
All of this is presumably why it has been been repeatedly shown that PUFA causes more DNL and weight gain than saturated fat, even with the same number of calories.
Long Chain PUFA
You may have heard that dietary PUFA from vegetable oil is elongated and used for PUFA signalling or whatever. It is.7
Around 1-3% of it. Another 10-20% is stored as is, mostly in visceral fat. The vast majority is used to cause reductive stress and DNL. Of course, that’s in rats, perhaps its more efficient in humans? Not so much.8

Conclusion
The vast majority of plant based PUFA eaten by humans is rapidly broken down into acetyl-CoA to be either used for immediate energy requirements or to be rebuilt into other fats.
The mitochondria doesn’t have a choice but to recycle PUFA into newly created fats via De Novo Lipogenesis. Since PUFA artificially stimulate PPAR alpha, they drive more fuel into the mitochondria than is being called for by the organism. Since PUFA fail to stimulate significant superoxide production, there is not enough oxygen to burn the fuel at this rate. The only possible outcome is a buildup of acetyl-CoA. When acetyl-CoA levels rise, mitochondria increase rates of De Novo Lipogenesis to rid them of the extra acetyl-CoA. The acetyl groups are turned into fat.
Carbon Recycling of PUFA happens exactly as you expect that it should.
- 1.Cunnane SC, Ryan MA, Nadeau CR, Bazinet RP, Musa-Veloso K, McCloy U. Why is carbon from some polyunsaturates extensively recycled into lipid synthesis? Lipids. Published online April 2003:477-484. doi:10.1007/s11745-003-1087-8
- 2.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. Published online April 29, 1997:4312-4317. doi:10.1073/pnas.94.9.4312
- 3.Burdge GC. Metabolism of α-linolenic acid in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids. Published online September 2006:161-168. doi:10.1016/j.plefa.2006.05.013
- 4.Jones PJH, Schoeller DA. Polyunsaturated: Saturated ratio of diet fat influences energy substrate utilization in the human. Metabolism. Published online February 1988:145-151. doi:10.1016/s0026-0495(98)90009-9
- 5.Taha AY, Ryan MA, Cunnane SC. Markedly raised intake of saturated and monounsaturated fatty acids in rats on a high-fat ketogenic diet does not inhibit carbon recycling of13C-α-linolenate. Lipids. Published online October 2006:933-935. doi:10.1007/s11745-006-5046-1
- 6.Burdge GC, Wootton SA. Conversion of α-linolenic acid to palmitic, palmitoleic, stearic and oleic acids in men and women. Prostaglandins, Leukotrienes and Essential Fatty Acids. Published online October 2003:283-290. doi:10.1016/s0952-3278(03)00111-x
- 7.Cunnane SC, Anderson MJ. The Majority of Dietary Linoleate in Growing Rats is β-Oxidized or Stored in Visceral Fat. The Journal of Nutrition. Published online January 1, 1997:146-152. doi:10.1093/jn/127.1.146
- 8.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. Published online August 2007:619-634. doi:10.1139/h07-034
