Introduction
This is the second article in my series about reductive stress. Check out the intro.
The terms oxidative stress and antioxidant have been thrown around a lot in recent decades, often without a clear understanding of their true nature. Oxidative stress is caused by an overproduction of superoxide and hydrogen peroxide or by a failure of the antioxidant system, ie glutathione and glutathione reductase.
Antioxidants are electron donors. They have the capacity to eliminate free radicals or to produce superoxide, depending on the context. In many cell systems, reductive stress – a buildup of cellular antioxidants glutathione, NADH and NADPH – is the root cause of oxidative stress.
Antioxidants
Free radicals are molecules with an unpaired electron. This makes them very reactive and they have the capacity to do damage to fats, proteins and DNA. Antioxidants are electron donors. Antioxidants donate the missing electron so that the free radical now has a pair of electrons.
Antioxidants have the magical ability to lose an electron without becoming highly reactive.
Many antioxidants can be recycled – they can gain the electron back, reverting to their original state. This is the “reduced” version of the molecule. We can remember this through the handy phrase OILRIG: Oxidation Is Losing, Reduction Is Gaining (electrons).
When we talk about oxidation states, we are talking about “redox” chemistry. Redox is just a funny name for the movement of electrons between molecules. We focus on the movement of electrons. Metabolism is mostly just moving electrons. Often, what is moving back and forth between the molecules is actually a hydrogen atom – a proton and an electron – but in redox terms the electron is what counts. So the molecule that is getting reduced gains an H. Whenever there is a dot after the molecule name, it means it is a free radical – it has an unpaired electron.
As an example, let’s look at a classic set of redox reactions – the neutralization of a free radical by Vitamin E and Vitamin C.1
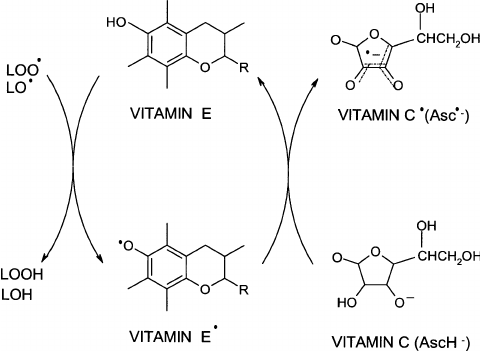
As you can see, Vitamin E (In the middle, reduced form on top, oxidized on bottom) and C (on the right, Reduced form on bottom, Oxidized from on top) both contain carbon rings. This isn’t by accident, ring shapes allow for “resonance” which is what allows them to take on the unpaired electron without themselves becoming highly reactive. The free radical – designated by LO(dot) at the upper left – gains an H to become LOH. (in this case L means lipid, Vitamin E is known for diffusing lipid peroxides) The vitamin E loses the H of its OH group which is replaced with a dot representing the unpaired electron. The original Vitamin E, the one on top, had one electron from the O of the OH group but the electron from the H was donated, leaving it with only one out of that pair. The bottom form of Vitamin E is the oxidized form. Oxidation Is Losing electrons.
In a redox reaction, by definition, one thing is always oxidized and the other one is reduced. Therefore, the LO(dot) becomes reduced as Vitamin E is oxidized. Reduction Is Gaining an electron. The same electron that Vitamin E lost from its OH bond is gained by LO(dot). Reduction of the free radical eliminates it.
Vitamin E cannot be oxidized a second time to eliminate another free radical. For that it needs reduced Vitamin C, which gives up an electron to regenerate reduced vitamin E. Vitamin C is oxidized to reduce vitamin E and winds up with the unpaired electron.
Antioxidant Summary
- OILRIG. Oxidation Is Losing, Reduction Is Gaining.
- When one thing is reduced, another one is oxidized.
- Antioxidants are electron donors.
- Antioxidants can be in either the oxidized or reduced states.
- To diffuse a free radical, antioxidants have to be in the reduced state.
- Reduced antioxidants can donate electrons.
Glutathione, NADPH and NADH
Let’s consider how glutathione – the body’s “master antioxidant” – diffuses peroxides.2 In this diagram glutathione is on the right. GSH is the reduced form and GSSH is the oxidized form. Reduced glutathione donates an electron to peroxides to diffuse them, becoming oxidized in the process. Reduced NADPH then donates an electron to oxidized glutathione to recycle its antioxidant potential, becoming oxidized to NADP+. NADP+ can again become the reduced NADPH by getting another electron from a cytosolic enzyme called glucose-6 phosphate dehydrogenase (G6PDH) in the pentose phosphate pathway.
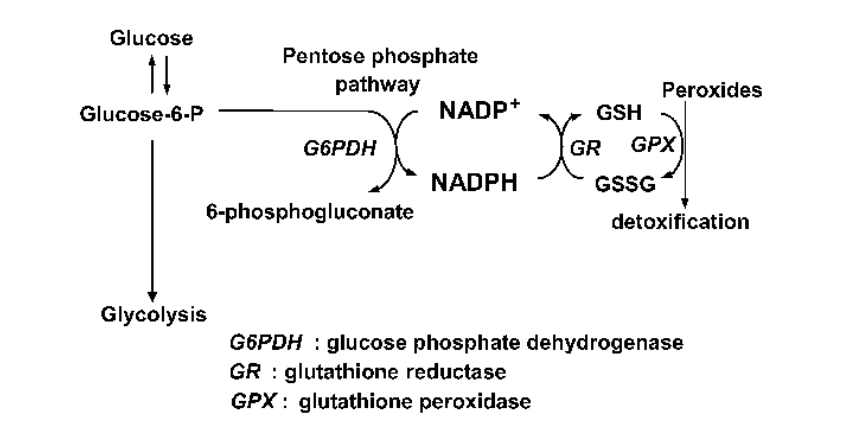
In this way, the pentose phosphate pathway is supplying a flow of electrons from glucose to NADP+ to oxidized glutathione to free radicals, diffusing them. It is an antioxidant pipeline.
NADH, the reduced form of NAD+, is also part of the antioxidant pipeline. A mitochondrial enzyme called NNT uses NADH to reduce NADP+ back to NADPH, allowing the NADPH to reduce an oxidized glutathione. The NADH/NNT pathway is a mitochondrial alternative to the pentose phosphate pathway.
Oxidative Stress
Oxidative stress is defined as an imbalance between free radical production in a cell and antioxidant activity. It can be measured in different ways, including by measuring the actual level of hydrogen peroxide buildup in a tissue or more commonly by measuring how much glutathione is in the reduced state vs. how much is in the oxidized state. If a higher amount of glutathione is in the oxidized state compared to a normal cell then the cell is said to be in oxidative stress.
Oxidative stress can be caused by either excessive superoxide production within the cell or by a failure of the antioxidant system. For instance, the chemical rotenone blocks Complex I of the mitochondrial electron transport chain. This does not allow the electrons to flow through the electron chain to create ATP. Instead, the electrons ping back out and wind up getting donated to O2 to form massive quantities of superoxide.3 In cells exposed to rotenone you’ll find a high concentration of hydrogen peroxide and the pool of glutathione will be very oxidized.
Reductive Stress
Reductive Stress is essentially the opposite of oxidative stress – a buildup of things in the reduced state. The things that build up in reductive stress are reduced glutathione, NADPH and NADH – the reduced form of NAD+. NAD+ is an important co-factor for diverse enzyme families such as the sirtuin protein deactylases and the PARP family of DNA repair enzymes.
Typically when we are talking about reductive stress what we mean is a high ratio of NADH/NAD+. NADH is the reduced form of NAD+. In obesity and diabetes, we see low levels of NAD+ and high levels of NADH: classic reductive stress.
Reductive stress is implicated in the pathology of diseases such as diabetic cardiomyopathy, coronary artery disease, Alzheimer’s Disease and metabolic syndrome4.
NADPH Oxidase (NOX)
Reduced antioxidants can donate electrons. This allows them to act as either oxidants or antioxidants depending on the context.
We have a family of enzymes who use this antioxidant pipeline to generate superoxide called NADPH Oxidases (NOX). They are expressed in all tissues.
NADPH oxidase takes the reducing power of NADPH – it’s ability to donate electrons, the very thing that is driving the antioxidant pipeline – and uses it to donate electrons to Oxygen, O2, creating superoxide. In fact, the two main sources of superoxide production in most cells are the mitochondrial electron transport chain (like in the rotenone example) and NADPH Oxidases.
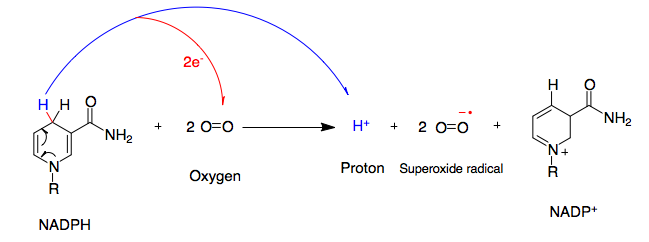
By <a href=”//commons.wikimedia.org/w/index.php?title=User:Marckhalaf&action=edit&redlink=1″ class=”new” title=”User:Marckhalaf (page does not exist)”>Marckhalaf</a> – <span class=”int-own-work” lang=”en”>Own work</span>, CC BY-SA 3.0, Link
What cells want is to be in oxidative balance – neither in oxidative stress or reductive stress. The ability to turn reducing power into free radicals is crucial for maintaining this balance.
Reductive Stress Creates Oxidative Stress
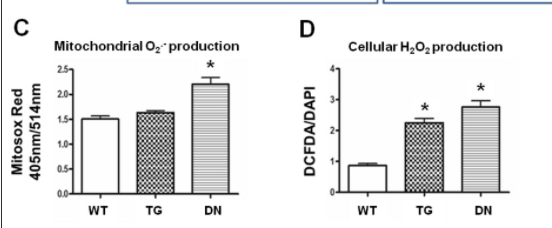
An experiment5 used heart-specific overexpression (TG) or deletion (DN) of NOX4 (NADPH Oxidase 4) in mice to examine its effects on Ischemia (heart attack) injury versus normal mice (WT). At baseline (BN) the mice without NOX4 activity had reductive stress as defined by a low NAD+/NADH ratio and by a buildup of reduced glutathione. The lack of ability to oxidize the antioxidant pool had thrown them into reductive stress. The mice who overexpressed NOX4 predictably had oxidative stress as defined by a relatively oxidized pool of glutathione. Oxidizing the pool of antioxidants beyond the biologically correct amount created oxidative stress.
The mouse hearts had the oxygen removed for 30 minutes, a procedure meant to stimulate a heart attack and which is known to cause oxidative stress. The result was the seeming paradox that the mice hearts lacking NOX4 activity that were in reductive stress – that had the highest levels of antioxidants (glutathione) and the biggest antioxidant defense pipelines (NADH, NADPH) – produced the MOST ROS in response to injury. In fact, those hearts – the ones that COULD NOT turn antioxidants directly into superoxide via the NOX4 enzyme – did not survive the experiment (heart rate of zero after oxygen was reintroduced, reintroducing oxygen is called reperfusion) whereas the normal hearts and the ones in oxidative stress survived. The hearts that started with the most reductive stress wound up with the most oxidative stress.
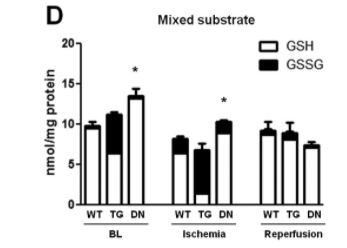
Here’s my explanation to the paradox. During respiration NADH carries electrons to the electron transport chain to get oxidized back to NAD+. When oxygen is removed from the mice hearts to stimulate a heart attack, oxidative respiration slows to zero. At this point, the electrons carried by NADH to complex I of the electron transport chain will ping back out, creating superoxide. The cells with the highest NAHD/NAD+ ratio have the strongest electron gradient and will continue handing electrons to Complex I to generate superoxide. As the cell becomes oxidized certain redox sensitive enzymes in glycolysis and the TCA cycle will stop working to prevent further generation of NADH (glyceraldehyde 3-phosphate dehydrogenase, aconitase). The cells with the largest reserves of reduced glutathione and NADPH will stay out of oxidative stress the longest, allowing glycolysis and the TCA cycle to continue reducing NAD+ to NADH, giving more fuel to the fire.
The huge antioxidant buffer in these cells has a huge potential for creating ROS and the lack of oxygen is the perfect context to set off a massive wave of ROS generation. It’s true that the cells that were overexpressing NOX4 and that were in oxidative stress before the oxygen deprivation ultimately experienced more oxidative stress than normal cells but those cells would have cut off the flow of glucose in much quicker and averted disaster. The cells in reductive stress had by far the most oxidative stress.
Conclusion
Oxidative balance in cells is crucial to the health of the organism. There are many different feedback loops operating. The ability to act as an antioxidant is the ability to generate superoxide. You can’t have one without the other. Antioxidants are electron donors whether the electron is donated to a free radical or to Oxygen.
The pro-oxidant NAPDH oxidase enzyme, NOX4, is crucial for providing oxidative balance. Eliminating it creates reductive stress, setting up the cell for oxidative catastrophe.
- 1.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. Published online November 2004:37-56. doi:10.1023/b:mcbi.0000049134.69131.89
- 2.Ogasawara Y, Funakoshi M, Ishii K. Determination of Reduced Nicotinamide Adenine Dinucleotide Phosphate Concentration Using High-Performance Liquid Chromatography with Fluorescence Detection: Ratio of the Reduced Form as a Biomarker of Oxidative Stress. Biological & Pharmaceutical Bulletin. Published online 2009:1819-1823. doi:10.1248/bpb.32.1819
- 3.Siddiqui MA, Ahmad J, Farshori NN, et al. Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol Cell Biochem. Published online August 21, 2013:59-69. doi:10.1007/s11010-013-1781-9
- 4.Pérez-Torres I, Guarner-Lans V, Rubio-Ruiz ME. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. IJMS. Published online October 5, 2017:2098. doi:10.3390/ijms18102098
- 5.Yu Q, Lee CF, Wang W, et al. Elimination of NADPH Oxidase Activity Promotes Reductive Stress and Sensitizes the Heart to Ischemic Injury. JAHA. Published online January 27, 2014. doi:10.1161/jaha.113.000555

This means that antioxidants alone won’t help much as long as we don’t get enough electrons from adequate amounts of glucose. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7070274
Throw away your antioxidants. Unless they’re lipoic acid, which is a pro-oxidant. Astaxanthin may also be a pro-oxidant.
Sounds like ray peat
Oh this is fascinating. I wonder if some of this is explanatory in the story of Sleep Apnea and its relationship to diabetes et al.
So take a Vitamin E and C daily to ward off coronary heart disease? Not trying to be funny. All of your articles have made me realize my body is a chemistry lab and have affected what I put in it for the better. Thanks
I’m not a fan of supplemental antioxidants. Your body is pretty good at balancing redox if you feed it right (no PUFA) and get your enzyme systems in line. I can’t imagine why supplemental antioxidants would be useful, at worst they will increase oxidative stress.
I’ve been thinking about is the role of gasses recently. Apparently Wim Hof breathing (which involves stopping breathing for at least 30 seconds) makes the blood alkaline, which makes sense because you stop getting O2, *the* source of oxidative power in the body, and build up CO2. This is also apparently very good for metabolism because people who do this can hike up freezing mountains in their underwear. Possibly a hormetic effect is involved there.
What about people with anemia? You might think that poor oxygen supply get less oxidative power from O2. But, maybe that backs up the ETC and ironically causes a lot of superoxide to be produced. People who live in the mountains basically have mild chronic hypoxia but are slim and live longer. Yet they also say sleep apnea causes obesity (I think that’s rather a common cause from poor diet).
I guess I just like the idea of messing with oxygen and seeing how the mitochondria responds. Maybe you could get an animal model of hyperoxigenation by overexpressing hemoglobin or doing those red blood cell infusions athletes do to cheat. Would that make them immune to PUFA-induced obesity? Susceptible?? So many questions.
There is quite a bit of literature on ischemia-reperfusion experiments. For my purposes I just thought this was a beautifully clean example of ROS from antioxidants.
Great per usual. Are we looking at an equilibriating system in which progressively larger O creates larger R; usually contained so that it damps down enough to allow cell life, but which can spiral to the point of death, leaving no R response possible.
The intrinsic inhibition of SDH (complex II) represents an evolutionary mechanism of diminishing ROS production in those organs, which have a wide range of functional activities and utilize long-chain fatty acids as a predominant substrate for energy production. Kidneys, however, do not possess this mechanism, and this raises the possibility that oxidative stress is not the primary pathogenic mechanism for the kidneys in people with metabolic syndrome.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9000033/
Hi great article. I think a piece of the puzzle is near infrared radiation and how it enables intracelluar production of melatonin, which act as a master antoxidant in clearing ROS within the cell.
/// Johan
I was wondering if you ever get concerned about the potential adverse consequences of inhibiting SCD1? (see article below) How do you know when you’ve gone too far? Maybe I missed it, but I couldn’t find any information on your Blog about the functions of SCD1, and how optimal health requires a tight regulation in the Goldilocks Zone (i.e., not too little, not too much, but just right). I hope you address this in the future. Thanks.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3042787/
Actually, I did a pretty detailed n=1 of this:
https://fireinabottle.net/sterculia-oil-brads-blood-numbers-and-safety/
I did a poorly documented n=1 with sterculia oil last year and coincidentally saw ALT and AST about 3x the upper reference range limits after several months of supplementation. CRP was also higher than I’ve ever experienced. It was enough for my doctor to get me an ultrasound and hematology appointment. The numbers returned to healthy levels after I stopped supplementing and we still aren’t sure what happened.
I’m starting up another sterculia oil trial soon, but this time I’m taking a lower dose and testing liver enzymes before I begin and then every 2-3 weeks to see how they respond.
Thank you Brad. Just layman’s idea, the main difference of PUFA beta oxidation I think is NADPH conversion to NADP+. So PUFA steals NADPH which cannot regenerate GSSG to GSH and can cause other deficiencies. Isn’t it more important than FADH deficiency? GSH deficiency I see as the main problem caused by PUFA.
I have an upcoming post where I explain how I think it is that PUFA causes the reductive stress. Essentially it’s lack of FADH2 input, which doesn’t create enough superoxide for glutathione reductase to recycle NAD+ via the NNT pathway.
Excellent.
But, you meant defuses, not diffuses.
Randomly just came across this comment on an old hyperlipid blog:
http://high-fat-nutrition.blogspot.com/2013/08/protons-so-far-some-sort-of-summary.html?showComment=1379138311158#c5703647742420336431
This stuff is above my pay grade so no idea if it’s obvious to you or interesting or what, but thought I’d mention it:
This is perhaps my all-time favorite lipid post!
Why is protein considered to be a nutrient that reduces obesity? Does your theory account for that? (Even Petro D. says he doesn’t know why protein seemingly decreases hunger.)
Protein does seem to have a satiating effect but I don’t consider lack of protein to be a cause of the obesity epidemic. I wrote recently about Thai rice farmers with a VERY high metabolic rate who were getting 85% of calories from carbs and ~10% from protein.