The idea of this series is that the underlying condition that drives obesity forward is reductive stress, as defined by the NAD+/NADH ratio being too low (not enough NAD+). CD38 is a membrane bound enzyme, expressed in most tissues, that increases throughout life, steadily reducing NAD+ levels1. It is the body’s “primary NADase”, the main enzyme that is stealing from the NAD+ pool. Along with dropping NAD+ comes decreased mitochondrial function and lowered metabolic rate.
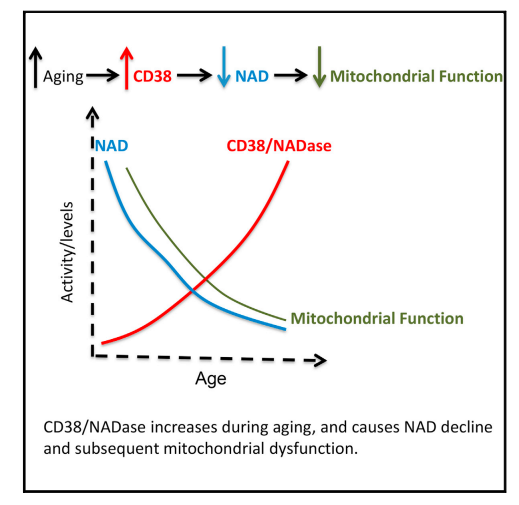
In past articles we’ve seen that the MAIN way we lose NAD+ is by converting it to NADH when it takes on an electron from our food. When NADH pushes that electron onto Complex I of the mitochondrial electron transport chain, the NADH converts back to NAD+.
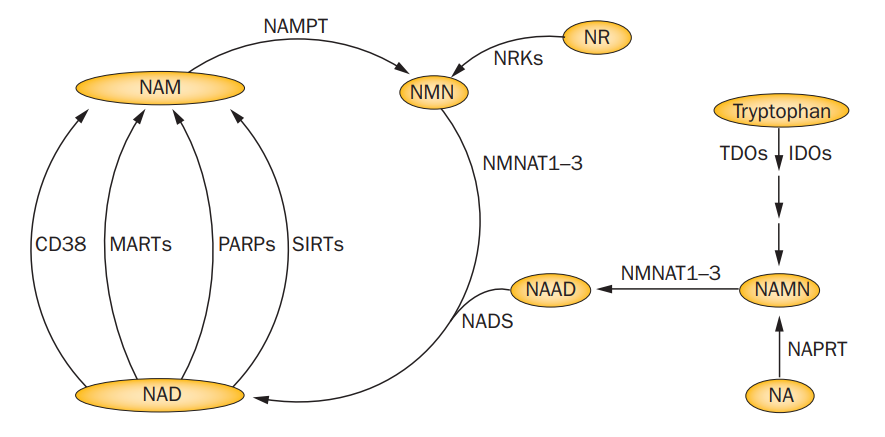
CD38 and other NADase are different. They actually release the nicotinamide part of the NAD+, requiring another enzyme called NAMPT to regenerate NAD+ through the “salvage pathway”. So there is a battle between CD38 and NAMPT to maintain NAD+ levels. When CD38 activity levels are high, NAD+ levels go down. Here is the whole pathway. NR is nicotinamide riboside, AKA niagen.
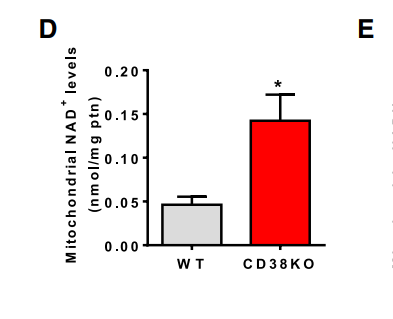
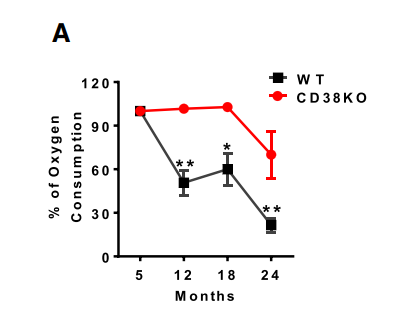
Mitochondria from cells lacking CD38 have very high levels of NAD+ and very high metabolic rates. Conversely, mitochondria from cells where CD38 is overexpressed have low levels of NAD+ and low metabolic rates.
Mice lacking CD38 don’t get fat
The REALLY interesting thing about CD38 is that if you remove the CD38 gene from mice, they are totally resistant to high-fat diet induced obesity!2
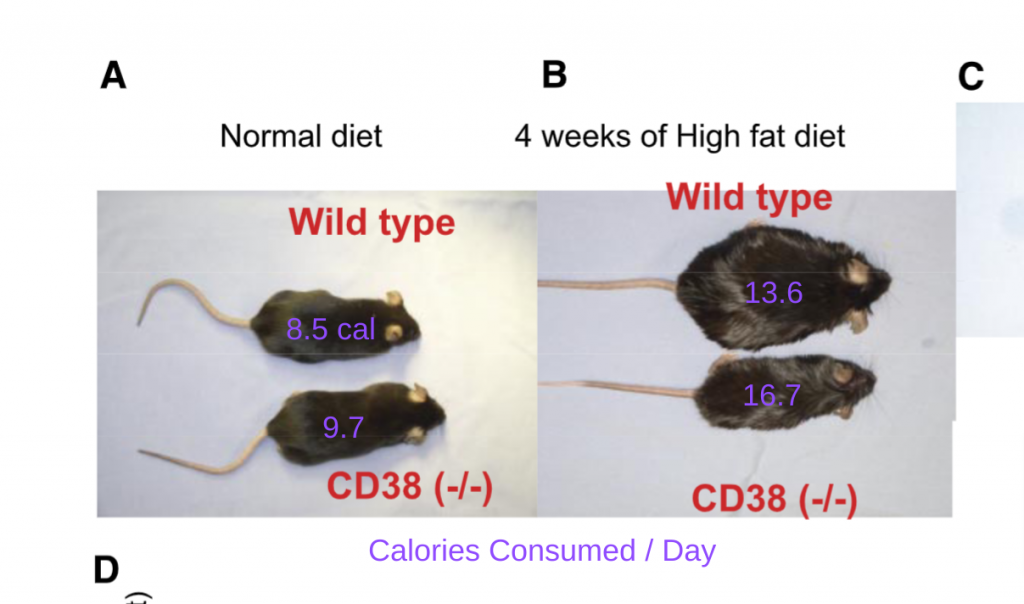
I added the daily caloric consumption to each group of mice on their back in violet. Which mouse over ate? The one who gorged on 16.7 calories per day and remained lean or the one who restricted itself to 13.4 calories per day and got fat? In addition to being gluttonous, the mice lacking CD38 were also lazy, showing much less spontaneous movement than normal mice. So the lean mice lacking CD38 maintained its waistline via an eat-more-move-less strategy.
It looks like mice who don’t get reductive stress don’t get fat!
LPS induced CD38 induction links inflammation and obesity and is reversed by restoring NAD+
There has been lots of discussion about obesity being caused by gut bacteria and its associated inflammation. It turns out that lipopolysaccharide (LPS), a compound released by bacteria into the bloodstream which stimulates the inflammatory response, upregulates CD383. You can make mice fat on a low fat, “chow” type lab diet that normally keeps mice thin by injecting them with LPS.4 Unfortunately this paper doesn’t follow the causality of LPS induced obesity all the way down to the NAD+/NADH level.
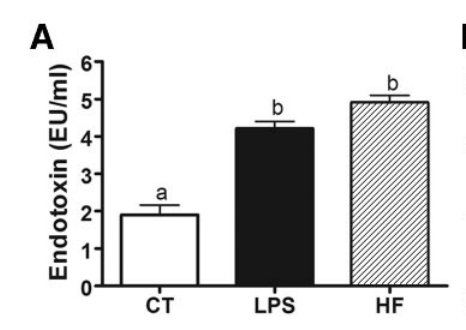
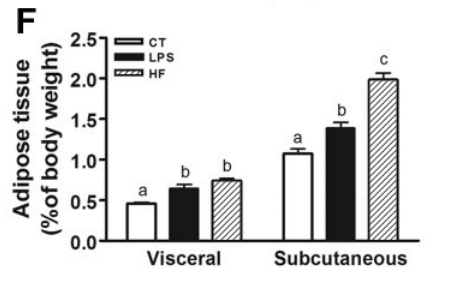
This one does, though!5 It is a very nicely done 2021 paper which clearly shows how LPS induces inflammation via upregulation of CD38 and a decline in NAD+. The paper goes on to show that supplements which can restore NAD+ can reduce the LPS-caused inflammation.
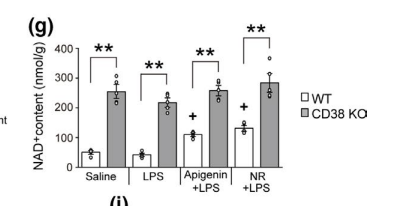
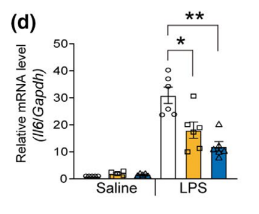
Mice were injected with LPS straight into their brains, which increased CD38 in the hippocampus and caused reductive stress and inflammation. Mice were given either nicotimamide riboside (NR) or a flavone CD38 inhibitor called apigenin. Both apigenin and NR increased the NAD+ levels and reduced inflammation as determined by levels of cytokines such as IL-6. The inflammatory response to LPS is mediated in large part by CD38 induced reductive stress.
ALA as a double check
I introduced alpha lipoic acid (ALA) in the last article not only because it is interesting as a supplement but because it also is useful to confirm our understanding of how this all works. ALA works primarily as an oxidant by oxidizing NADH and increasing the amount of NAD+. With that in mind, ALA should ALSO reverse the effects of LPS induced inflammation. It does. This paper6 concludes, “ ALA inhibits LPS-induced monocyte activation and acute inflammatory responses in vitro and in vivo”.
Restoring NAD+
I’ve shown evidence that three different approaches to raising NAD+ levels are effective in combating LPS-induced inflammation.
- ALA restores NAD+ via the direct oxidation of NADH.
- Nicotinamide Riboside (AKA Niagen) provides an NAD+ precursor which gets around the bottleneck of NAMPT activity.
- Apigenin inhibits CD38, the primary thief of NAD+.
I believe in thinking about these problems from a pathway perspective. Perhaps ALA will do you little good if your CD38 activity is high. It may make some sense to take these together.
Summary
CD38 is the primary enzyme which is stealing your NAD+, putting you into reductive stress. CD38 is greatly increased in response to the bacterial endotoxin LPS. LPS is far from the ONLY thing that increases CD38, lots more of those to come! LPS does, however, fill in one puzzle piece for us, “What is the link between LPS and obesity?”
In this series I’ve already written that reductive stress is the root cause of obesity and now we see how bacterial endotoxin can cause it. The reason the last two articles (this and the previous one) were released in this order is that I wanted to explain the roles of alpha lipoic acid (ACA) and CD38. Now that that is done, we can go ahead and start talking about how vegetable oil leads to reductive stresss…..
Check back in!
- 1.Camacho-Pereira J, Tarragó MG, Chini CCS, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metabolism. Published online June 2016:1127-1139. doi:10.1016/j.cmet.2016.05.006
- 2.Barbosa MTP, Soares SM, Novak CM, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet‐induced obesity. FASEB j. Published online June 21, 2007:3629-3639. doi:10.1096/fj.07-8290com
- 3.Amici SA, Young NA, Narvaez-Miranda J, et al. CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions. Front Immunol. Published online July 10, 2018. doi:10.3389/fimmu.2018.01593
- 4.Cani PD, Amar J, Iglesias MA, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. Published online July 1, 2007:1761-1772. doi:10.2337/db06-1491
- 5.Roboon J, Hattori T, Ishii H, et al. Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide‐induced microglial and astrocytic neuroinflammation by increasing NAD +. J Neurochem. Published online May 9, 2021:311-327. doi:10.1111/jnc.15367
- 6.Zhang WJ, Wei H, Hagen T, Frei B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA. Published online March 6, 2007:4077-4082. doi:10.1073/pnas.0700305104

Just bought some ALA and Apigenin. Curious to see if it makes any difference.
TCD was working for about a month (lost an average of 2 pounds a week), but now, it crept back up. Went from 261 in early February to 246 in March and back up to 259 as of yesterday. According to my smart scale, that weight gain is all fat.
I’ve been doing all i can do reduce SCD1, and following the protocols here (adding straight stearic acid or tallow or cacao butter to my OMAD, drinking pu’erh tea and jiaogulan, cutting way back on coffee, keeping sugar to a minimum, avoiding pufa and mufa) and kept gaining.
If these supplements don’t help, i think i’m gonna have to get back to keto…. Which will suck even more now that i know bacon and eggs are off the table
I hope they help!
Brad, I just wanted to comment to say thank you for all the research you’ve been doing. I was just thinking yesterday about how brilliant the croissant diet was. In one experiment, you proved basically all the mainstream explanations for obesity to be bunk—no calorie restriction, no eating windows, you mixeds fats and refined carbs, included sugar and alcohol, and ate hardly any protein or fiber. Nowadays you are on a roll with the NAD+ stuff. It’s all very valuable!
Quick question: how much do you suppose it matters that ROS can directly oxidize PUFA? You and Peter have suggested in the past that the difference in ROS production is because of FADH2:NADH ratios. Experiments like exogenous succinate show pretty clearly that the ratio matters a lot. But is it possible part of the difference is because PUFA is “soaking up” ROS? Theoretically the resulting products can be more dangeous than ROS, because we have the enzymes to diffuse superoxide and H2O2, while lipid peroxides are more varied and presumably need a bunch of different enzymes to neutralize them all. I feel like there’s a huge disconnect in the anti-oil crowd, with one side saying ROS stuff and the others talking about inflammation… so I wonder if that could be a direct connection.
Hi! PUFA CAN be oxidized by ROS and doing so is bad. I agree that lipid peroxides are generally bad. However, my focus is on more fundamental aspects of how PUFA affect mitochondrial dynamics.
Brad
From what I’ve gathered Quercetin also inhibits CD38 aswell as activate AMPK (aswell as a seeming host of budding research indicating other benefits of consumption). It isnt however very bioavailable and is recommended to be consumed with Bromelain and Vitamin C, perhaps an issue for ROS activity?
Why would lps inhibit mitochondrial function through reductive stress? Seems like an evolutionary based process……as long as not prolonged.
What, if any, explanation for NAD and mito function decrease with age ? Is it functional in some sort of way?
What do you think the healthy function of CD38 is supposed to be? It seems more plausible that CD38 has some role it is supposed to play that goes out of whack.
I found a marginally comprehensible (to the layperson like me) 2019 review that makes several comments on the possible roles of CD38 in the conclusion, including that “it is possible that CD38 may have a key role in the immune response to bacterial infections.”
https://www.frontiersin.org/articles/10.3389/fimmu.2019.01187/full
That fits with the rough idea that metabolic and immune dysfunction are coupled, but I’m in over my head on this.
Hi Tom!
Sorry for the much delayed response. CD38 may have some very general role in inflammation. It is also possible that decreasing NAD+ IS an immune function: https://academic.oup.com/femsre/article-abstract/45/6/fuab037/6315328?redirectedFrom=fulltext
Hi brad;
Is it true that even-chain saturated fatty acid are ketogenic and odd-chain saturated fatty acid are anti-ketogenic (glucogenic)?!
I don’t know!
Hi Brad, interesting article. I came across this discussion on Long Covid which centers on the role NAD+ https://www.youtube.com/watch?v=ZFPleh6z7io
Dr Ade Wentzel has been treating long covid via increasing NAD+ using the Preiss-Handler pathway, instead of via the salvage pathway as he’s found that more effective in treating long covid. He does mention the role of CD38 in the discussion. As someone who’s got long covid I’ve been using his suggestions for increasing NAD+ via Nicotinic acid (Niacin). In addition I’ve been using stearic acid and ALA plus cutting out PUFA’s and following a high fat diet. I’ve mainly been doing this to help long covid but i’ve noticed that since adding in the Niacin it’s speeded up the weight loss. I’d be interested in your take on Dr Wentzel’s approach to this. With regard to the Apigenin have you any idea on most effective dosage/timing?
I don’t have time to watch the vid ATM, but it sounds like we are of a similar mindset. The anecdote about niacin and the Preiss-Handler pathway speeding weight loss for you is interesting.
Brad
Hello again,
Sorry to double comment on this post, before you’ve even had a chance to approve my first one.
Ive been taking ALA and Apigenin, but haven’t added Niagen.
I was wondering if the desired results could potentially be achieved with the supplements i have.
If not, is it a good idea to take other NAD+ precursors or a straight NAD+ supplement?
I’m sure it’s obvious, but the reason I’m asking is because i’m already spending too much on supplements (Apigenin, ALA, Vitamin D, multivitamin, stearic acid), and Niagen is pretty expensive.
Thanks for all the information
Sorry for the slow comment moderation. It’s actually NOT obvious. I lean towards the side of combining ALA with Niagen for NAD+. Apigenin should help, but I do have some concerns with flavones in general. The stearic acid and Vit D should act independently of the others.
Are you familiar with Nuchido Time+ which combines ALA, Nicotinamide, Sophora Japonica (quercetin, rutin and troxrutin), Parsley (apigenin), EGCG, Vitamin C, Zinc, and Black Pepper Extract?
They claim to increase NAD+ levels by 242% in blood cells in 16 days.
From what I understand there is some dispute as to whether NAD+ levels in the blood is relevant compared to tissue levels but it does confirm much of what you have written here.
Very very interesting…
So how much apigenin would be needed?
I am already taking 100mg/day before bed but maybe need more and more often?
Same for NR or NMN which I’ve tried both but they’re pretty expensive 🙁
Cheers from the UK.
If I am thinking about this correctly, NOX1, Nox3, Nox4, Nox5, and NOS2 are all NADPH producers. There are two specific locations on NOS2 that upregulate its expression quite a bit. So controlling this may be helpful – one of the feeders of NOX is the arginine pathway – so L – Lysine can limit the arginine as fuel.
Luteolin also slows down CD38.
And NQO1 is the enzyme responsible for returning it back to NAD+, and when mutated can be upregulated by Pau D Arco. This particular enzyme is critical for detoxification of a number of solvents, herbicides, and pesticides.
NAD+ activates SIRT3, and SIRT3 activates AMPK, so you can see how clearly the link is to being in mTOR instead of AMPK with low NAD+. Fortunately SIRT3 is upregulated by Berberine and Resveratrol.
I think you have this backwards: the Nox enzymes are CONSUMERS of NADPH, converting it back to NADP+ and releasing superoxide.
Hi Brad, thank you for all you do! Is it possible to get the power point presentation file from your Future of Fat Summit video? thank you
Just emailed it to you.
Not sure the best place to post on this. Saw this pubmed report on Berberine and wanted to see your take on it. Degraded muscle synthesis certainly sounds like a negative.
https://pubmed.ncbi.nlm.nih.gov/20522589/?fs=e&s=cl
I need to update some parts of the blog, I’ve grown less interested in berberine, although it still has potential uses. I think all of the plant-based polyphenols may have some drawbacks.