This post serves as a summary of the key points of my video, “How Olive Oil Makes You Fat”. Much more evidence is presented in the video, including epidemiological data in humans, feeding trials in rodents and pigs and stuff about grizzly bears. Plus jokes.
This paper looked at oxidized linoleic acid metabolites AKA oxlams AKA oxylipins AKA oxylipids in actual, walking-around lean and obese humans. This is as close as you’ll find to a roadmap to obesity. The red pen is my addition.
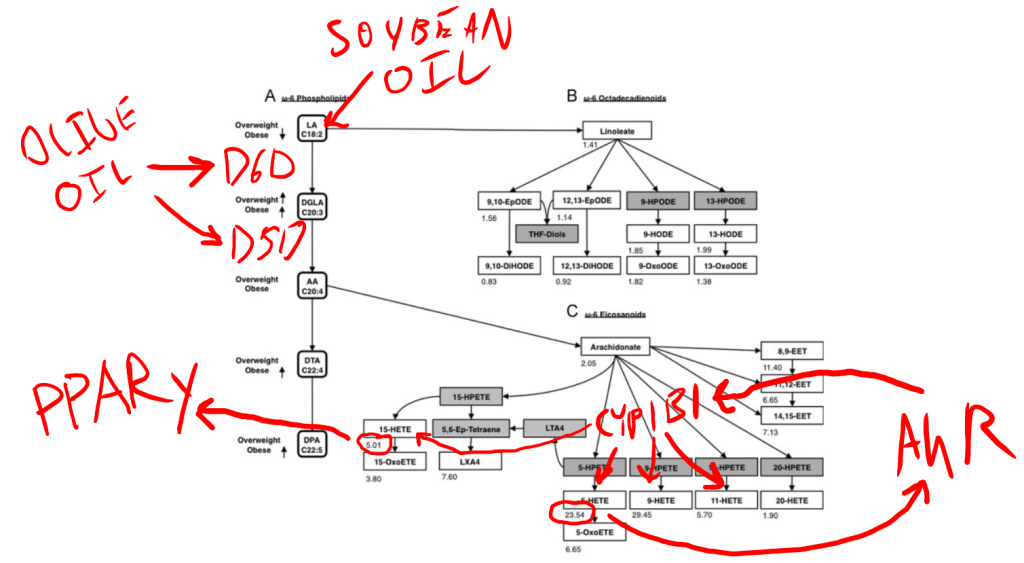
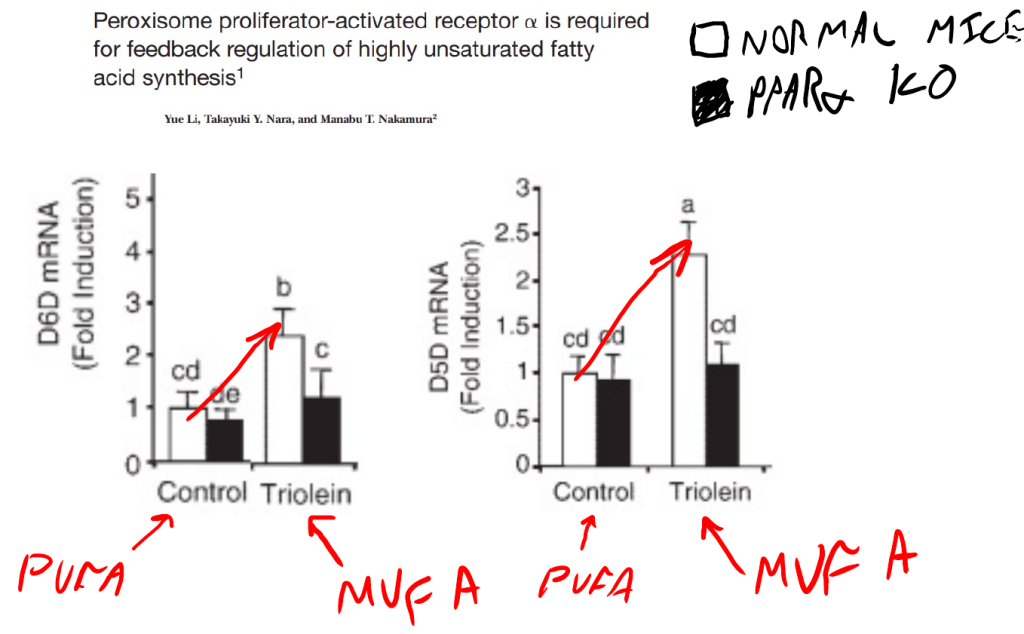
In obese humans, 5-HETE – a linoleic acid (LA C18:2 – from soybean oil) metabolite – is increased 24 times versus lean humans. That’s no typo. 5-HETE is 24 times higher in obese humans. 5-HETE is produced from arachidonic acid (AA C20:4). The enzymes D6D and D5D control the rate at which linoleic acid is converted to arachidonic acid. The expression of D6D and D5D is controlled by dietary oleic acid. If you swap the 7% of the normal lab mouse diet that is soybean oil out with pure oleic acid, the expression of D6D and D5D more than double. This happens because oleic acid activates PPAR alpha.
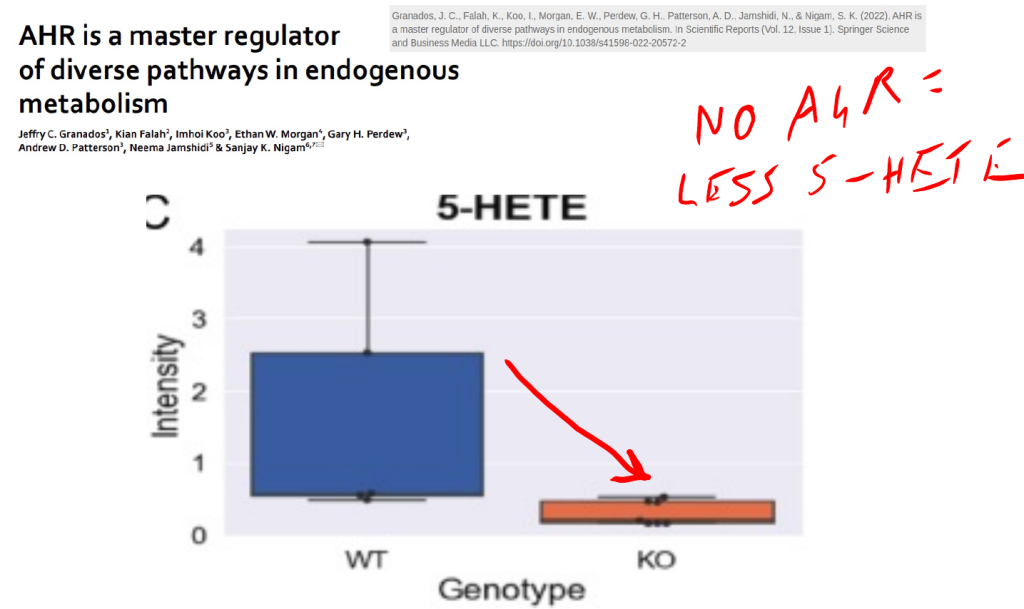
Arachidonic acid is converted to 5-HETE by the enzyme CYP1B1, whose expression is controlled by the aryl hydrocarbon receptor (AhR). This is likely a positive feedback loop, with 5-HETE activating the AhR to produce more 5-HETE. If you remove the AhR from a mouse, 5-HETE levels plunge.
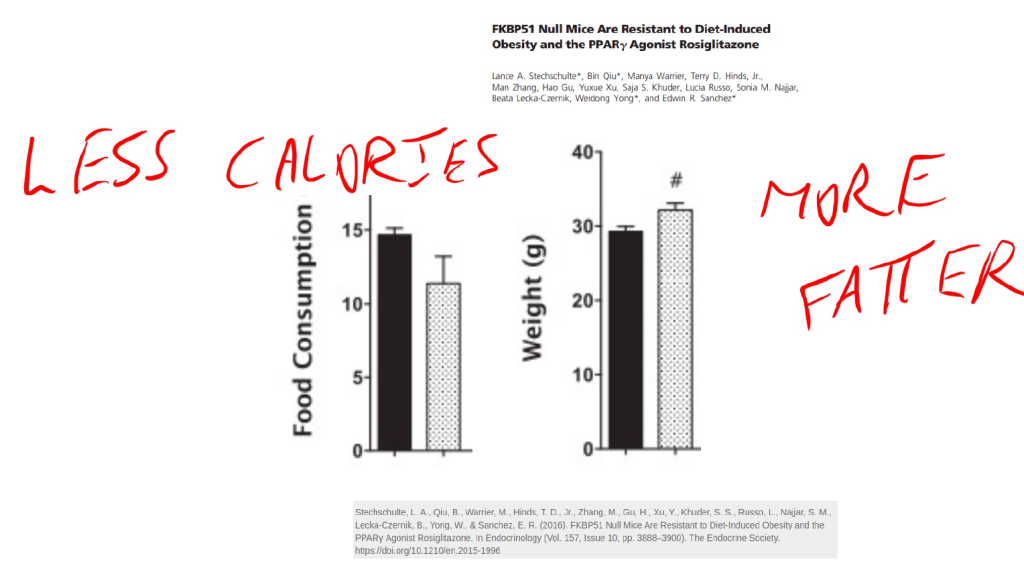
Another product of CYP1B1 is 15-HETE, which is elevated 5 fold in obesity. 15-HETE activates PPAR gamma, similar to the pharmaceutical rosiglitazone. If you give mice rosiglitazone, they will gain weight while eating less calories.
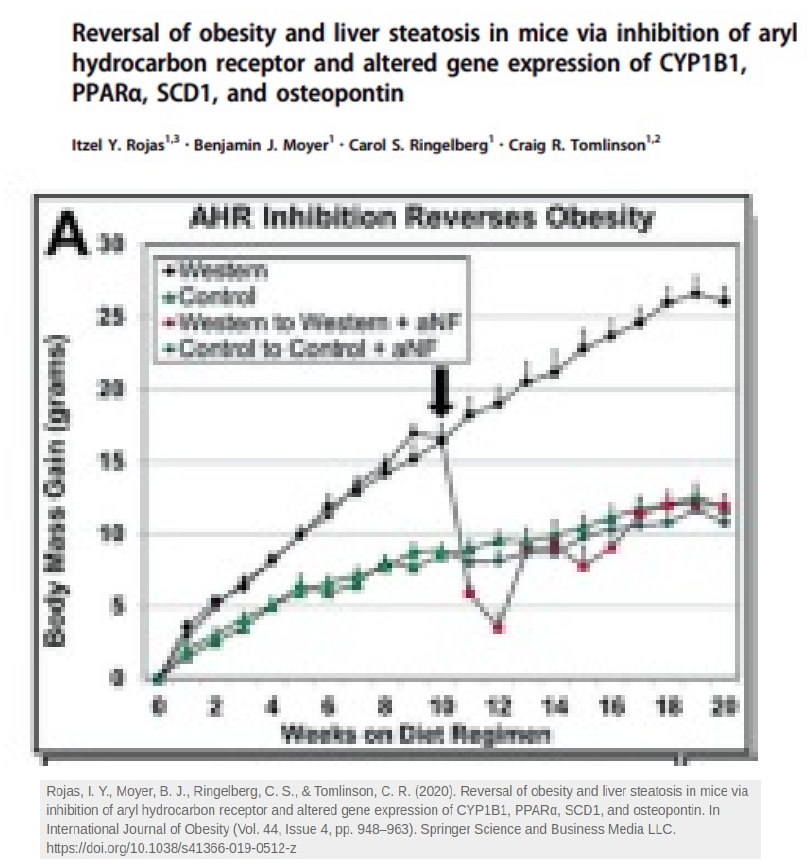
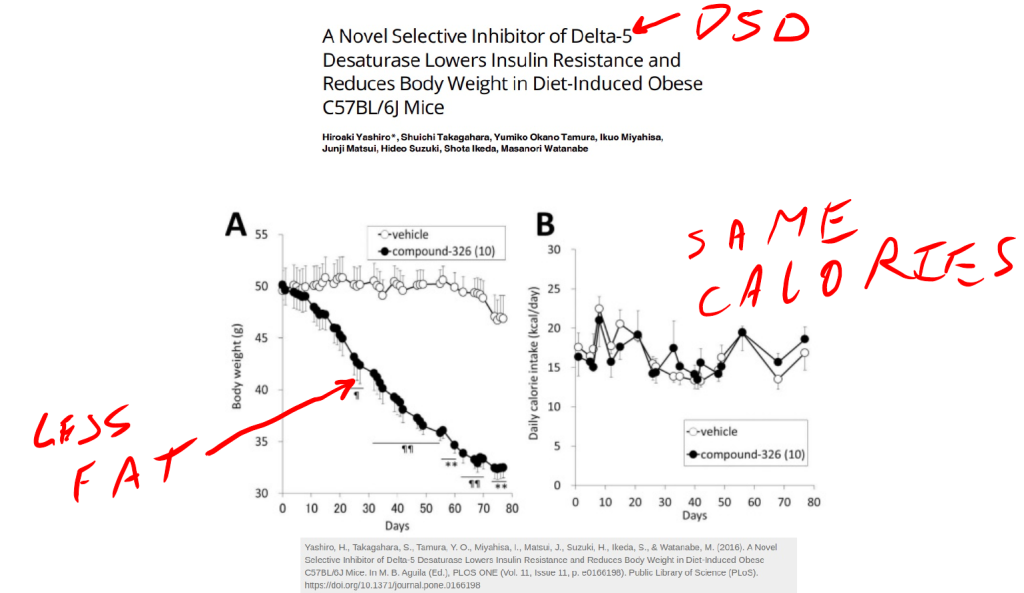
If you give mice a pharmaceutical that blocks either the AhR or D5D, you break the cycle and reverse obesity.
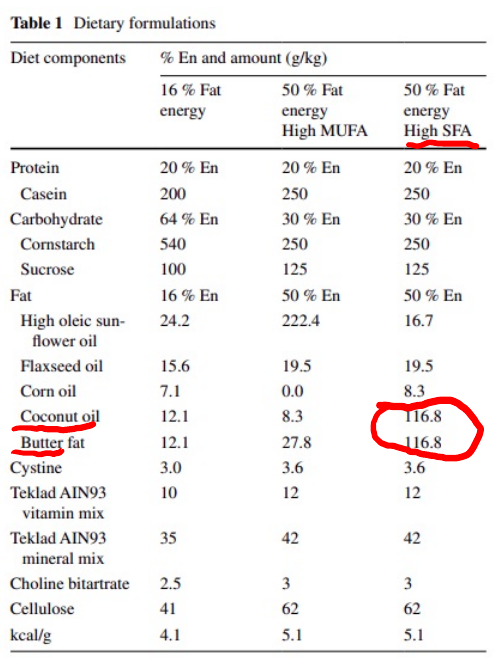
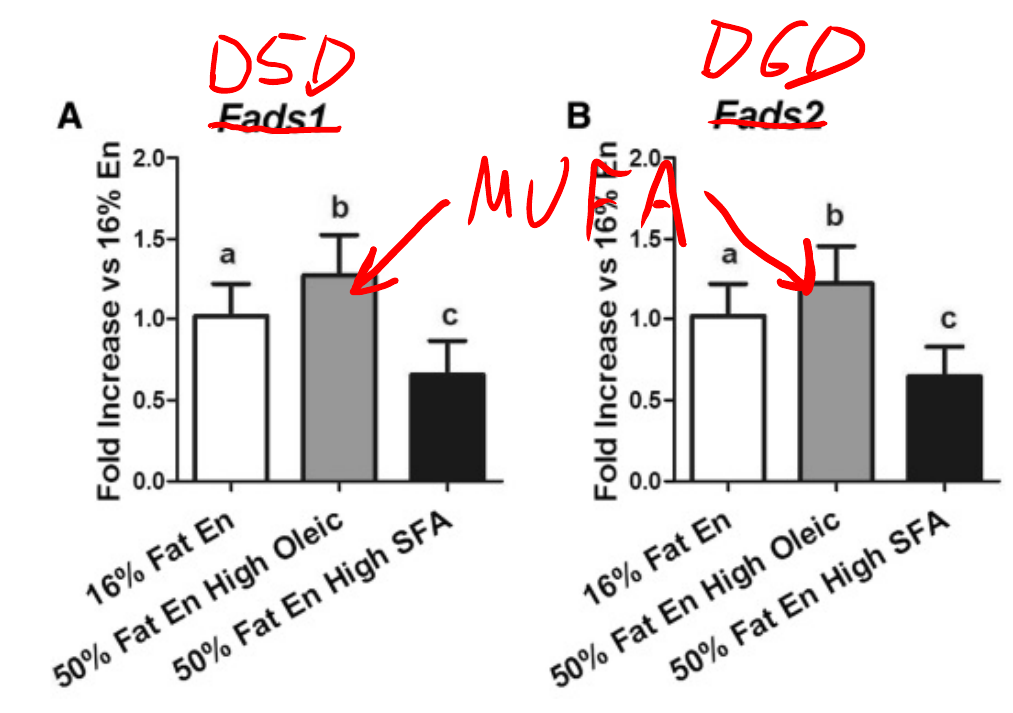
Mice fed a high MUFA diet have increased D6D and D5D activity, whereas mice fed a high saturated fat diet have lowered D6D and D5D activity.

This study looked at elderly nursing home patients with normal blood glucose control (the control group). All meals were prepared in the cafeteria, so the diet was completely controlled. In the baseline diet, all cooking was done with high linoleic sunflower oil and in the test diet all added fat was virgin olive oil. Fat profiles of the membrane phospholipids of patients were reported, showing increased D6D activity after the switch to olive oil.

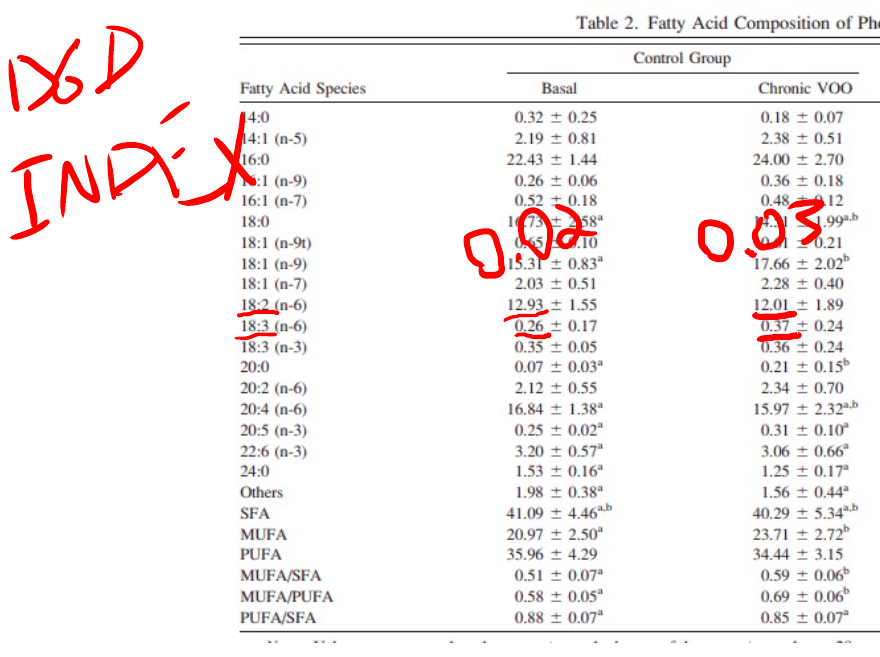
An indirect measure of enzyme activity can be calculated using the product-to-precursor ratio of an enzyme. If enzyme B converts compound A to compound C, when its activity increases, compound C will go up and compound A will go down. D6D converts linoleic acid (18:2 n-6) to GLA (18:3 n-6).

See how under the olive oil diet, linoleic acid drops and GLA increases? This means that more linoleic acid is being converted to arachidonic acid.
We can see that the olive oil consuming patients are doing more De Novo Lipogenesis than when sunflower was being consumed since the DNL index rises. The DNL index is the ratio of palmitic acid (16:0) to linoleic acid. When D6D is very active, 15-HETE increases, activating PPAR gamma and the lipogenic enzymes. The end product of DNL is palmitic acid. So if linoleic acid drops and palmitic acid increases, DNL is happening. This can be obscured by the diet. For instance, butter is a large source of palmitic acid and might push the ratio higher in the absence of DNL. In this case, the olive oil diet is low in palmitic acid, meaning that the increase is almost certainly from DNL.
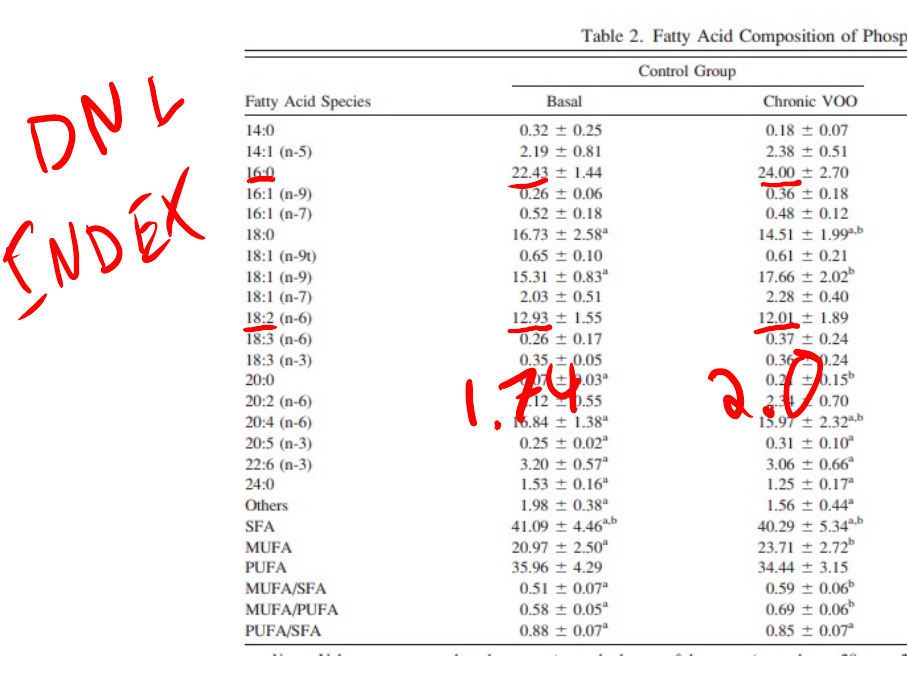
If you REALLY wanted to stimulate DNL you would add a diet based on MUFA to drive D6D and D5D that was sufficiently high in PUFA. Something like canola oil.

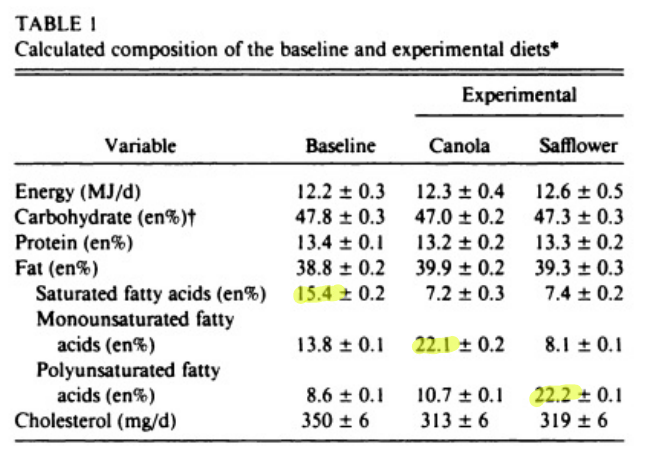
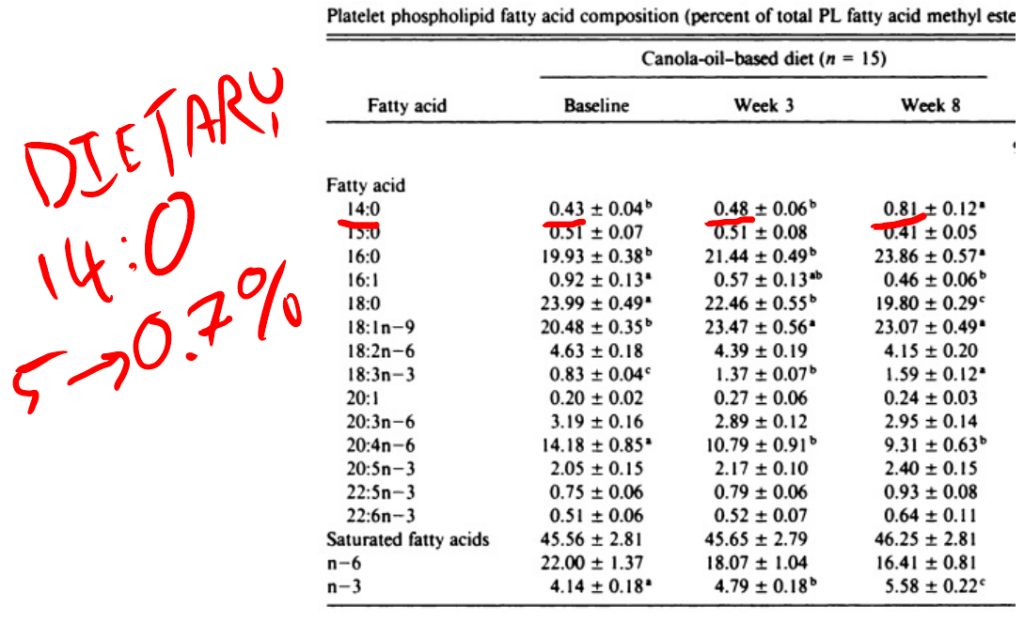
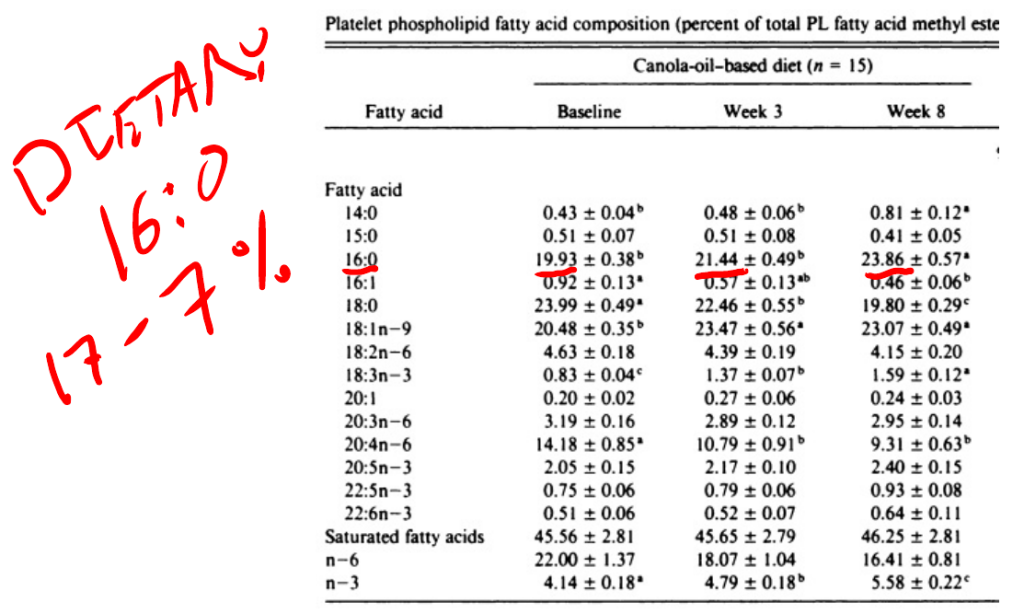
In this study young men were fed a butter based baseline diet (with some seed oils) for three weeks, then switched to a high linoleic acid diet or a canola oil diet for eight weeks. All meals were prepared in a metabolic kitchen. The safflower oil increased DNL as you can see by the rises in both palmitic acid and myristic acid (14:0) – another end-product of DNL that is a useful indicator.
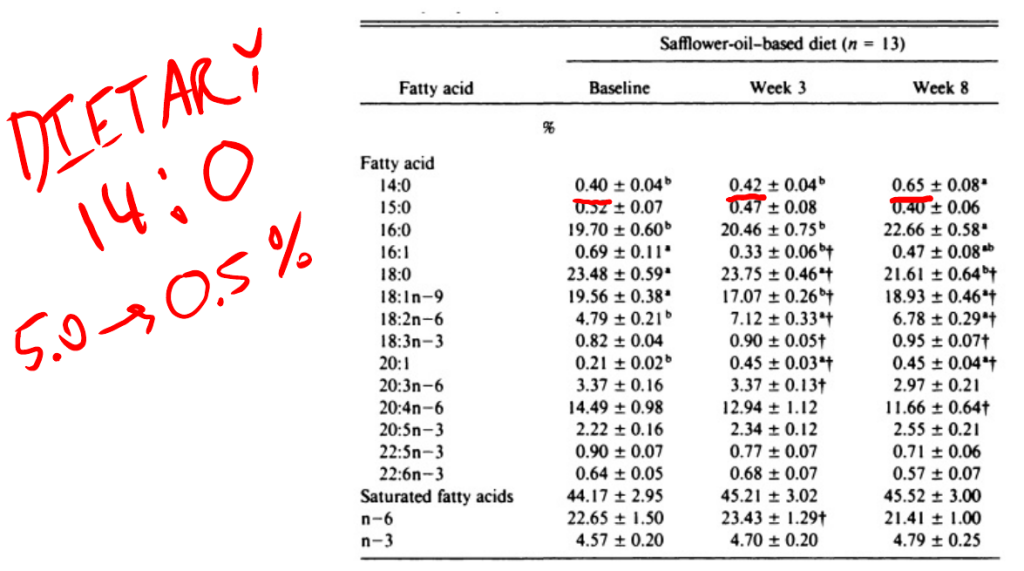
Myristic acid rose over 60% in a diet with massively reduced myristic acid composition. The myristic acid has to be coming from increased DNL relative to the butter based diet. Myristic acid is the single best indicator of DNL in actual, walking-around humans.
The level of myristic acid explodes in the canola-oil based diet in parallel with palmitic acid. Again, the diets are low in both palmitic acid and especially myristic acid. The increases of both are significantly steeper on the canola oil based diet than on the safflower oil based diet.
Conclusion
Oxidized metabolites of linoleic acid cause obesity. The best way to oxidize linoleic acid is with dietary oleic acid. A MUFA based fat that is sufficiently high in PUFA is the most fattening fat. It’s worth pointing out that this exactly describes the diet most often used in the lab to induce obesity in rodents. You know, the one they call “saturated fat”.
Kwon, J., Snook, J., Wardlaw, G., & Hwang, D. (1991). Effects of diets high in saturated fatty acids, canola oil, or safflower oil on platelet function, thromboxane B2 formation, and fatty acid composition of platelet phospholipids. In The American Journal of Clinical Nutrition (Vol. 54, Issue 2, pp. 351–358). Elsevier BV. https://doi.org/10.1093/ajcn/54.2.351
Perona, J. S., Vogler, O., Sanchez-Dominguez, J. M., Montero, E., Escriba, P. V., & Ruiz-Gutierrez, V. (2007). Consumption of Virgin Olive Oil Influences Membrane Lipid Composition and Regulates Intracellular Signaling in Elderly Adults With Type 2 Diabetes Mellitus. In The Journals of Gerontology Series A: Biological Sciences and Medical Sciences (Vol. 62, Issue 3, pp. 256–263). Oxford University Press (OUP). https://doi.org/10.1093/gerona/62.3.256
Pickens, C. A., Sordillo, L. M., Comstock, S. S., Harris, W. S., Hortos, K., Kovan, B., & Fenton, J. I. (2015). Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. In Prostaglandins, Leukotrienes and Essential Fatty Acids (Vol. 95, pp. 31–40). Elsevier BV. https://doi.org/10.1016/j.plefa.2014.12.001
Stechschulte, L. A., Qiu, B., Warrier, M., Hinds, T. D., Jr., Zhang, M., Gu, H., Xu, Y., Khuder, S. S., Russo, L., Najjar, S. M., Lecka-Czernik, B., Yong, W., & Sanchez, E. R. (2016). FKBP51 Null Mice Are Resistant to Diet-Induced Obesity and the PPARγ Agonist Rosiglitazone. In Endocrinology (Vol. 157, Issue 10, pp. 3888–3900). The Endocrine Society. https://doi.org/10.1210/en.2015-1996

But …….
the Mashed article update in 2023 doesn’t mention this article which gives a broader perspective than only pointing to cooking oil being the source of the problem
https://www.theguardian.com/education/2001/aug/25/research.highereducation
Coffee?
It’s all about the succinate
BTW, Brad, I miss the days when this was a blog, not a vlog. I know that I’m probably outnumbered in this, but I can read faster than I can listen, and I get more out of text than from video.
Given the numbers and the drift, I understand that it’s probably easier to make a video than edit a blog post. For me, even 10 minutes is still tl;dw(atch). And video can’t be searched.
Thanks for what you are doing, and putting up with the complaints 🙂
The release of triolein from the liver is interesting in designing snacks-
https://www.nature.com/articles/ijo2017231
“Triglycerides cross the blood–brain barrier and induce central leptin and insulin receptor resistance” W A Banks et al. 2017; Nature: Clinical Studies and Practice. (International Journal of Obesity volume 42, pages391–397 (2018)).
Resistance at the brain receptors for leptin and insulin has been associated with increased feeding, obesity and cognitive impairments. The causal agent for central resistance is unknown but could be derived from the blood.
The radioactive triglyceride triolein readily crossed the BBB and centrally administered triolein and peripherally administered lipids induced in vivo leptin and/or insulin resistance at hypothalamic receptors. Central triolein blocked the satiety effect of centrally administered leptin.