This paper from Finland is literally the greatest nutrition paper I’ve ever seen. (Lankinen, 2019)
It’s long been known that high plasma levels of linoleic acid predict low diabetes risk. (Kroger, 2010) Furthermore, plasma levels of linoleic acid are a biomarker for intake. At this point people of a certain predisposition will say, “See! I told you PUFA prevents diabetes!”
But diet is a rather poor predictor of plasma linoleic acid levels. According to the EPIC-POTSDAM study, (Kroger, 2010) dietary linoleic acid only accounts for 21% of the variation seen in plasma levels.
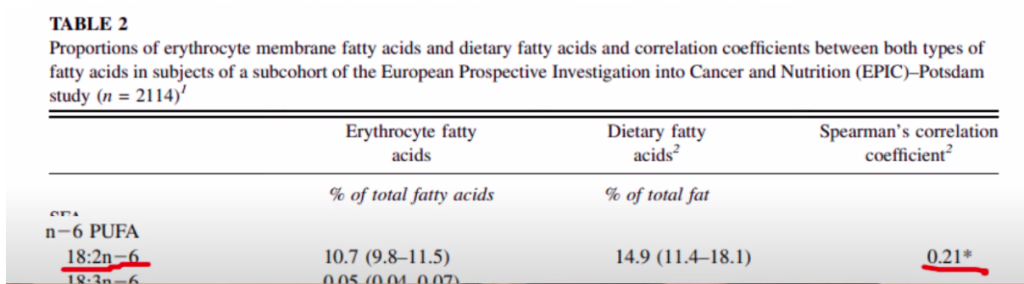
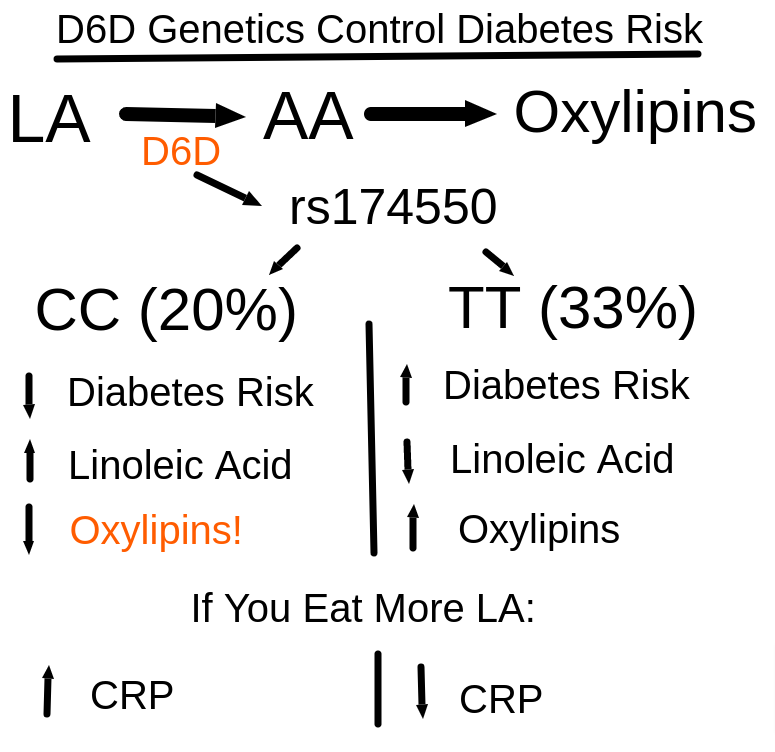
A 2019 Mendelian Randomization study clarified the issue: the best predictor of plasma linoleic acid levels are variants of the gene that encodes the enzyme Delta 6 Desaturase (D6D). (Zhao, 2018) D6D is the rate limiting enzyme that controls the conversion of linoleic acid to arachidonic acid and then ultimately to oxylipins – oxidized, bioactive linoleic acid metabolites.

So if you have high D6D activity, you’ll have low levels of linoleic acid since you made the rest into oxylipins!
Watch the video!
This is the video version of this post, if you prefer. There is some extra info in the blog post, however. The stuff about 12-HETE and PPAR Gamma. But you do you.
D6D Activity Level Predicts Diabetes Risk
Enzymes leave footprints. The pathway I’ve shown above is simplified. In fat D6D converts linoleic acid (LA) to Gamma Linolenic Acid (GLA; 18:3n6). Two more enzymes are required to convert GLA to arachidonic acid, but D6D is the rate limiting one for the pathway. If you compare the ratio of the product of the enzyme (GLA) to the precursor (LA), the ratio gives you an indirect look at the relative activity of the enzyme. This is the D6D Desaturase Index.
This is the same concept I’ve talked about with the SCD1 desaturase index.
Here is how well the D6D desaturase index predicts the risk of getting diabetes within 5 years:
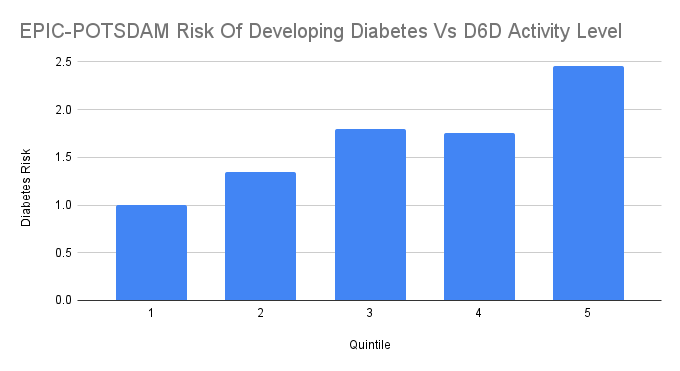
Furthermore, we can say that high D6D activity CAUSES diabetes according to several mendelian randomization studies. It’s not just a correlation. (Borges, 2022; Jager, 2020; Abbasi, 2015; Kroger, 2010)
The Finland Paper
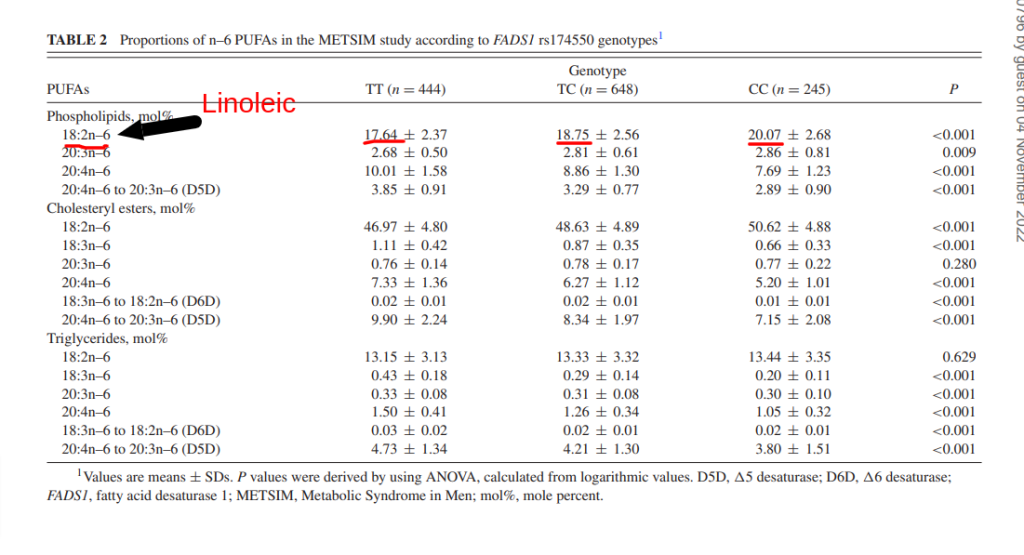
One of the things that makes the paper (Lankinen, 2019) extraordinary is that it was done in Finland, which has very low baseline consumption of PUFA. The participants were split into two groups – one having two copies of the genetic version of D6D with the lowest activity level (lowest diabetes risk – CC) and the second group having the version of the gene with the highest activity level (Highest diabetes risk – TT). In the population at large, 33% were TT (bad), 20% were CC (good) and around half were TC (in the middle).
As you can see, baseline levels of linoleic acid were predicted by the genetics of the participants:
They took baseline data from the participants in each group. Then they put them on a diet which raised their linoleic acid intake from around 10 grams per day to a little over 30 grams per day. Thirty grams is comparable to what an average American would eat.
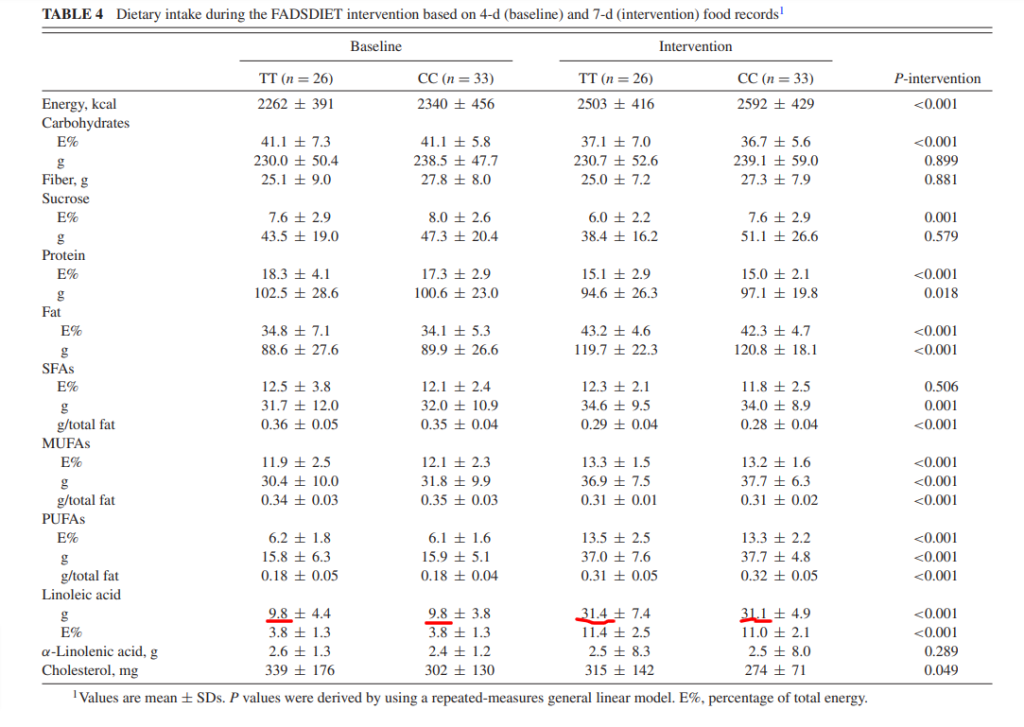
Not ONLY did the researchers measure TOTAL oxylipins, they actually measured the levels of 70 individual oxylipins!! (In the supplemental data.)
This study gives us a great look at what really happens with oxylipins as the diet changes from a more traditional one to an American style diet where we can see the interaction between diet and genetics!! Here is what happened with oxylipin levels:
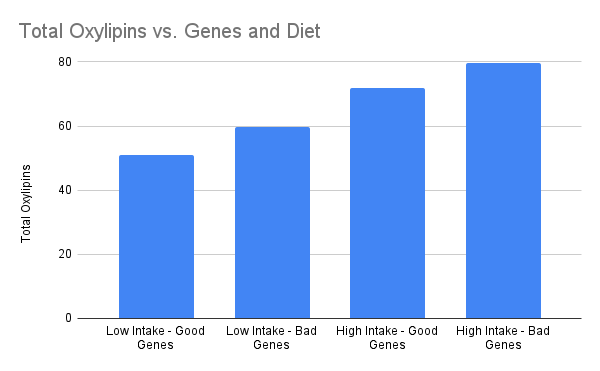
Inflammation
The great irony of this paper is that the people with the most active copy of the D6D gene (TT) had their CRP numbers (a marker of inflammation) LOWERED by increasing linoleic acid consumption. The opposite was true for those with the LOWEST expression of D6D. So in this case, if you took linoleic acid and it lowered inflammation, that is a very BAD thing because it suggests you have the worst genetics.
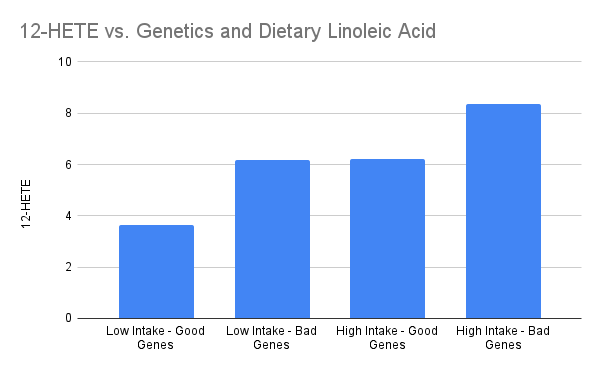
I find the inverse correlation between CRP and diabetes risk of particular interest because PPAR Gamma is known to be activated by oxidized PUFA, is very involved in lipid metabolism (lipogensis) and is known to lower CRP( Kong, 2011). Let’s take a look at the specific oxylipin 12-HETE, which is made by CYP1B1 (Shoeib, 2020) – an enzyme whose expression is controlled by the aryl hydrocarbon receptor – and which is a known activator of PPAR Gamma (Han, 2014).
Linoleic Acid Consumption Is Correlated With Diabetes Rates in Western Europe
After seeing how low baseline consumption of linoleic acid was in Finland, I graphed the correlation between the sum of sunflower oil and soybean oil consumption and diabetes rates in Western Europe. As you can see, they correlate. This doesn’t prove anything, but it’s food for thought. Most of the Nordic countries also eat some canola oil, which is not shown.
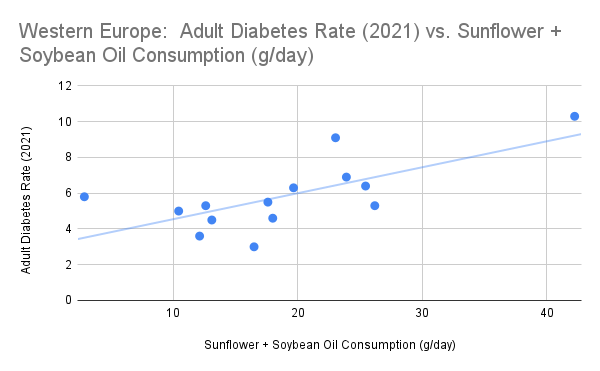
Here is the raw data. Data sources are FAOSTAT (https://www.fao.org/faostat/en/) and The Diabetes Atlas, 10th Edition (https://diabetesatlas.org/).
| Sunflower + Soybean Oil Consumption (g/day, 2017) | Adult Diabetes Rate (2021) | |
| Denmark | 12.62 | 5.3 |
| Finland | 2.88 | 5.8 |
| Iceland | 17.61 | 5.5 |
| Netherlands | 13.11 | 4.5 |
| Norway | 12.13 | 3.6 |
| Sweden | 10.45 | 5 |
| France | 26.19 | 5.3 |
| Germany | 23.91 | 6.9 |
| Ireland | 16.5 | 3 |
| Italy | 25.45 | 6.4 |
| Portugal | 23.04 | 9.1 |
| Spain | 42.23 | 10.3 |
| Switzerland | 18 | 4.6 |
| United Kingdom of Great Britain and Northern Ireland | 19.67 | 6.3 |
Note About D5D
If you look at these papers, you might notice that high levels of an enzyme called D5D – which is the enzyme that actually creates arachidonic acid – is associated with LOW diabetes risk. This is because the variants of D5D and D6D are in tight “linkage disequilibrium”. That means that the genes are right next to each. As it works out, if you get the copy of D6D that has HIGH activity you are overwhelmingly likely to get the copy of D5D that has LOW activity. So high D5D activity predicts LOW D6D activity and is accociated with low diabetes risk because D6D is the rate limiting enzyme and therefore the one associated with the risk.
Conclusions
This study should end debate about whether or not increasing linoleic acid consumption increases body levels of oxylipins in humans. It does. We also know that the role of D6D activity is likely CAUSAL in the development of diabetes. That strongly suggests that oxylipins cause diabetes because D6D only has one job.
Furthermore, there is a significant interaction between genetics and diet, which probably goes a long ways towards explaining why some people (20%) seem to do fine on a high PUFA diet whereas some have real problems (33%) and some are in the middle (50%).
As to whether PUFA is inflammatory – well, that’s a bit complicated! What we CAN say is that it changes inflammatory markers. Sometimes for the worse, sometimes for the better. In your case, I hope that linoleic acid increases your CRP because that means you have good genetics. But then if you have good genetics you probably won’t read this.
Citations
Abbasi, A. (2015). Mendelian randomization studies of biomarkers and type 2 diabetes. In Endocrine Connections (Vol. 4, Issue 4, pp. 249–260). Bioscientifica. https://doi.org/10.1530/ec-15-0087
Borges, M. C., Haycock, P., Zheng, J., Hemani, G., Howe, L. J., Schmidt, A. F., Staley, J. R., Thomas Lumbers, R., Henry, A., Lemaitre, R. N., Gaunt, T. R., Holmes, M. V., Smith, G. D., Hingorani, A. D., & Lawlor, D. A. (2022). The impact of fatty acids biosynthesis on the risk of cardiovascular diseases in Europeans and East Asians: A Mendelian randomization study. Cold Spring Harbor Laboratory. https://doi.org/10.1101/2022.04.17.22269308
HAN, J., SUN, L., XU, Y., LIANG, H., & CHENG, Y. (2014). Activation of PPARγ by 12/15-lipoxygenase during cerebral ischemia-reperfusion injury. In International Journal of Molecular Medicine (Vol. 35, Issue 1, pp. 195–201). Spandidos Publications. https://doi.org/10.3892/ijmm.2014.1998
Jäger, S., Cuadrat, R., Hoffmann, P., Wittenbecher, C., & Schulze, M. B. (2020). Desaturase Activity and the Risk of Type 2 Diabetes and Coronary Artery Disease: A Mendelian Randomization Study. In Nutrients (Vol. 12, Issue 8, p. 2261). MDPI AG. https://doi.org/10.3390/nu12082261
Kong, A. P. S., Yamasaki, A., Ozaki, R., Saito, H., Asami, T., Ohwada, S., Ko, G. T. C., Wong, C. K., Leung, G. T. C., Lee, K. F., Yeung, C. Y., & Chan, J. C. N. (2011). A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. In Diabetes, Obesity and Metabolism (Vol. 13, Issue 9, pp. 806–813). Wiley. https://doi.org/10.1111/j.1463-1326.2011.01411.x
Kröger, J., Zietemann, V., Enzenbach, C., Weikert, C., Jansen, E. H., Döring, F., Joost, H.-G., Boeing, H., & Schulze, M. B. (2010). Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. In The American Journal of Clinical Nutrition (Vol. 93, Issue 1, pp. 127–142). Oxford University Press (OUP). https://doi.org/10.3945/ajcn.110.005447
Lankinen, M. A., Fauland, A., Shimizu, B., Ågren, J., Wheelock, C. E., Laakso, M., Schwab, U., & Pihlajamäki, J. (2019). Inflammatory response to dietary linoleic acid depends on FADS1 genotype. In The American Journal of Clinical Nutrition (Vol. 109, Issue 1, pp. 165–175). Oxford University Press (OUP). https://doi.org/10.1093/ajcn/nqy287
Shoieb, S. M., & El-Kadi, A. O. S. (2020). Resveratrol attenuates angiotensin II-induced cellular hypertrophy through the inhibition of CYP1B1 and the cardiotoxic mid-chain HETE metabolites. In Molecular and Cellular Biochemistry (Vol. 471, Issues 1–2, pp. 165–176). Springer Science and Business Media LLC. https://doi.org/10.1007/s11010-020-03777-9
Zhao, J. V., & Schooling, C. M. (2018). Role of linoleic acid in autoimmune disorders: a Mendelian randomisation study. In Annals of the Rheumatic Diseases (Vol. 78, Issue 5, pp. 711–713). BMJ. https://doi.org/10.1136/annrheumdis-2018-214519

How can we see the papers you site at the beginning of the article. The links all say that the session has timed out.
Seen this…
https://pubmed.ncbi.nlm.nih.gov/23112819/
Brad, I cannot find any clear information on popcorn, I know corn has linoleic acid but does dry aired poppcorn have it as well, if it does is it the same amount? Or is the linoleic acid in the “shell” of the seed only on corn and the “white” popcorn does not have linoleic acid? Cheers, John S