I’ve been writing a lot about reductive stress and its role at the heart of both oxidative stress and obesity. I’m aware that the term reductive stress isn’t terribly intuitive. It’s a wonky, scientific term and calling it “the opposite of oxidative stress” really does little to aid in understanding for non-redox scientists.
I’ve been looking for the right analogy and I think the one that makes the most sense is the flooded engine.
At the end of this series I will discuss what you can do about reductive stress now that you know the problem.
Flooding The Engine
For the Christmas of 1987, my parents bought us kids an ATV. That morning we put my little brother on it, who was 5 or 6. We recorded him with our betamax video camera as he drove directly into a tree. Hit it square on. Of course he didn’t have a helmet on, it was the eighties! Don’t worry, he was fine. He didn’t know how to shift, so he never got out of first gear. It was a slow motion collision.
Anyway, the electric start on the ATV was broken, so you had to start it by pulling the cord, then reaching over the seat to give it some gas with the throttle that was controlled with your right hand thumb. You had to give it just enough gas. If you gave it too much, the engine would flood and you’d have to wait half an hour for the unburnt fuel to leak back out of the pistons.
Quickly I learned other situations that could flood it, for instance punching the gas going up a hill in too high of a gear. The engine would make that tell tale bogging sound and black smoke would come out of the tailpipe.
A google search tells us, “A flooded engine is an internal combustion engine that has been fed an excessively rich air-fuel mixture that cannot be ignited.”
The problem is too much fuel and not enough oxygen.
Acylcarnitines are the black smoke
A metabolic hallmark of obesity and diabetes are high circulating levels of acylcarnitines.1 The enzyme that controls fat flow into the mitochondria is called carnitine palmitoyltransferase (CPT1). This enzyme takes a free fatty acid (palmitate) and transfers it (that’s the transferase part) to a chaperone molecule called carnitine. Free fatty acids contain an acyl group on one end, so a molecule of fat attached to carnitine is an acylcarnitine. Got it?
If you don’t care to memorize all of that, just remember that acylcarnitines are unburnt fuel. Once fat is transferred to carnitine, it is supposed to be sent into the mitochondria to be burned.
Once inside the mitochondria, the acyl group is transferred from carnitine to another chaperone molecule called Coenzyme A (CoA) to become acyl-CoA. Fats are broken down in a process called beta-oxidation. Every round of beta oxidation produces an acetyl-CoA and a shortened acyl-CoA. Most fats are 16 or 18 carbons in length and an acetyl group has two carbons so it takes 8 or 9 rounds of beta-oxidation to turn a fat all of the way into acetyl-CoA. One molecule of fat makes 8 or 9 acetyl-CoA, each of which take 3 NAD+ to be fully burned.
So acyl-carnitines are used to transport fat into (or out of) the mitochondria for burning and acyl-CoA and acetyl-CoA are active fuel in the pistons.
Still following? The pathway is: acylcarnitine -> acyl-CoA -> acetyl-CoA -> TCA cycle
Let’s look at the full cycle of beta-oxidation. You don’t have to memorize it, the key point is to look at where NAD+ is used in the cycle. NAD+ is needed to move the fatty acyl-CoA past the hydroxyacyl-CoA step.
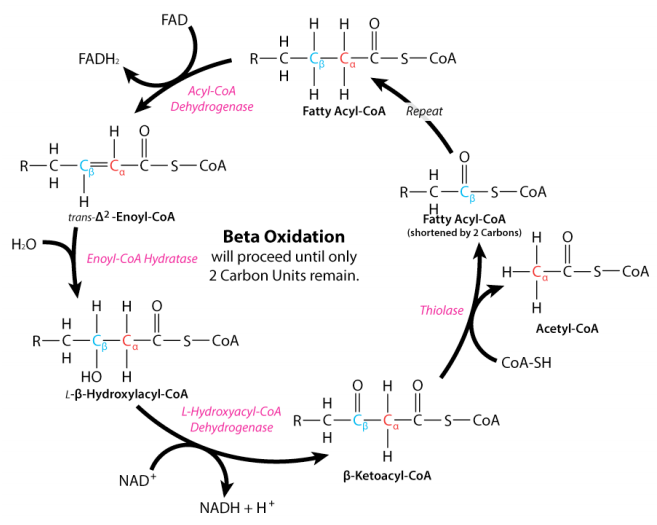
If the mitochondria is in a state of reductive stress, meaning that NAD+ is in too low of a supply, this step will go slowly and hydroxyacyl-CoA will start to build up. Ultimately, some of it will get transferred back to carnitine and be transported back out of the mitochondria. This is the black smoke coming out of the tailpipe: unburnt fuel is leaking out of the pistons.
Let’s look at the relative amounts of hydroxyacylcarnitines in lean adults, obese adults and diabetic adults.
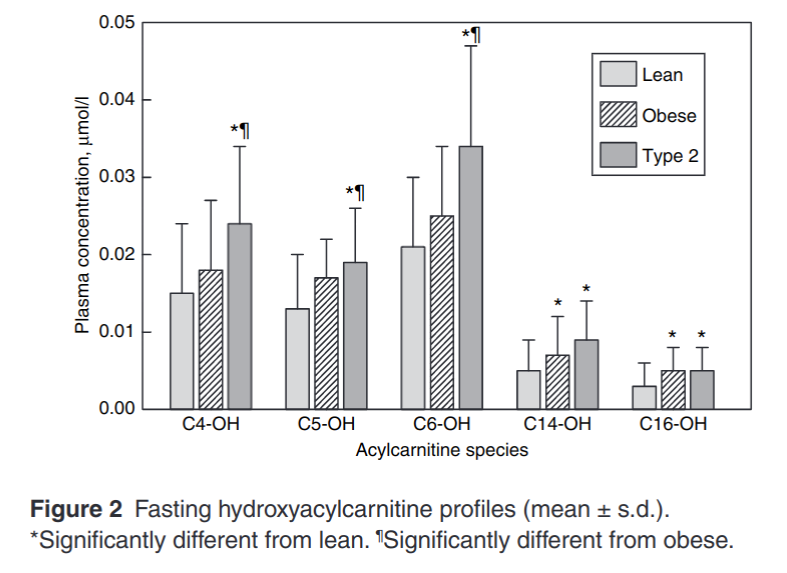
C6-OH is a 6 carbon hydroxyacylcarnitine. When you see a buildup of C6-OH, this is a molecule of fat that was chosen to be burned by CPT1. It was most likely 18 carbons in length to start with. It made its way into the mitochondria, got transferred to CoA and went through 6 rounds of beta-oxidation. At that point, NAD+ was limiting, so it hung around long enough to be transferred back to carnitine and was ultimately transferred into the bloodstream.
Another thing that is elevated in obesity is circulating acetylcarnitine.2 Acetyl means two carbons. This is an indicator that acetyl-CoA is also lingering in the mitochondria, not burning. Eventually the acetyl groups are transferred back to carnitine and leave the mitochondria. Not enough oxygen (NAD+). More smoke out of the tailpipe.
In an ATV piston, the fuel is ignited by mixing directly with oxygen. In the mitochondria, NAD+ plays the role of oxygen. If there is not enough NAD+, the air-fuel mixture will be too rich and the fuel won’t burn. It looks like the obese and diabetic humans have flooded engines to me.
Acetyl-CoA drives reductive stress and mitochondrial dysfunction
Reductive stress doesn’t come from nowhere. Ultimately it comes from your food. Fat, carbohydrate, ethanol and some amino acids can all be converted to acetyl-CoA. The pool of acetyl-CoA is the source of the high-energy electrons which reduce NAD+ to NADH. As I’ve said, it takes three NAD+ to burn a molecule of acetyl-CoA. The more acetyl-CoA there is, the faster NAD+ is converted to NADH.
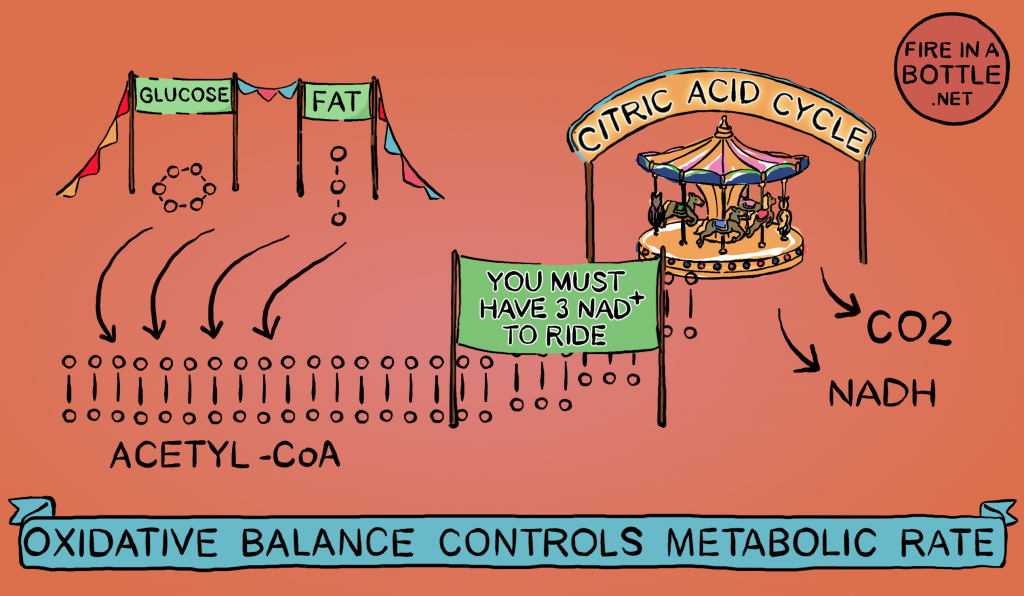
Therefore, a buildup of acetyl-CoA is one of the sources of reductive stress.
This is doubly interesting because when one is in significant reductive stress, mitochondrial enzymes become acetylated and turned off. Notice that you can’t spell acetylated without acetyl. That’s right, the buildup of acetyl-CoA is the very thing driving mitochondrial dysfunction. This is not a coincidence. The acetyl groups spontaneously (without needing an enzyme) affix themselves to mitochondrial enzymes3. This happens faster as the concentration of acetyl-CoA rises.
Compounding the problem, the Sirtuin enzymes – deacetylases that take acetyl groups off of enzymes – are dependent on NAD+ to do their job. A mitochondria with high levels of acetyl-CoA and low levels of NAD+ – AKA a mitochondria under reductive stress – will have mitochondrial dysfunction.
Alpha Lipoic Acid
Readers of my reductive stress articles might be wondering when I will point out the effect of lipoic acid on flooding your engine. Alpha Lipoic Acid (ALA) is an oxidant that oxidizes NADH to NAD+, relieving reductive stress. Look at the effect of a single IV injection of ALA into lean or diabetic rats on liver acetyl-CoA levels. (reference 8) Mind you, ALA is well absorbed orally, there’s no need to inject it.
| Liver Acetyl-CoA (nmol/g tissue) | |
| Non-diabetic | 30.45 |
| Diabetic | 53.55 |
| Diabetic plus ALA | 18.46 |
If you can add enough oxygen (supply enough oxidized NAD+), you can burn the fuel. You can unflood the engine and relieve reductive stress.
Reductive Stress And Torpor
I’ve written a lot about the parallels between human obesity and mammalian torpor. Torpor is the metabolic state that mammals use to lower their body temperature to hibernate.
In the lab when you want an animal to go into torpor, you lower the temperature and remove their food. The effect of fasting is a rapid rise in PPAR alpha – the master regulator of fat burning. PPAR alpha upregulates CPT1, which I’m sure you remember is the enzyme that controls the rate at which fat enters the mitochondria.
Then in 2012 it was demonstrated that all you had to do to send mice into torpor was to give them a torporish diet containing 18% of calories each from corn oil and lard and give them a pharmaceutical that activated PPAR alpha4. No fasting required!
NOTE: This is not meant as a blanket indictment of stimulating PPAR alpha. Later in torpor/obesity, PPAR alpha is downregulated and at that point stimulating it is probably sensible. More on this in later articles.
We can see that torpid animals are in reductive stress because they have highly acetylated mitochondrial enzymes5 and because when they want to get out of torpor, they surge production of sirtuin deacetylases6.
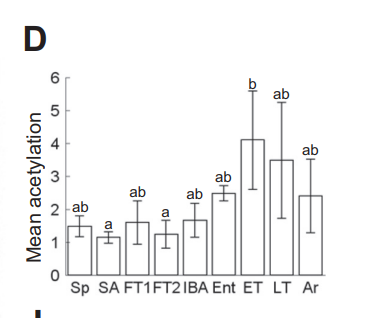
All that you have to do to induce torpor is to flood the mitochondria with fat. Fat is very rich in acetyl groups, providing three times as many, gram per gram, as glucose. The surge of acetyl-CoA runs down NAD+ levels and floods their little engines.
Lamb Suet
There’s a catch, though. Not all fat induces torpor equally. It was shown way back in 1987 that sheep suet (kidney fat) fed chipmunks couldn’t get into as deep of a state of torpor and they awoke more quickly than chipmunks fed sunflower oil7. The chipmunks fed the saturated fat continued to burn more oxygen compared to the PUFA fed rodents, maintaining a higher metabolic rate.
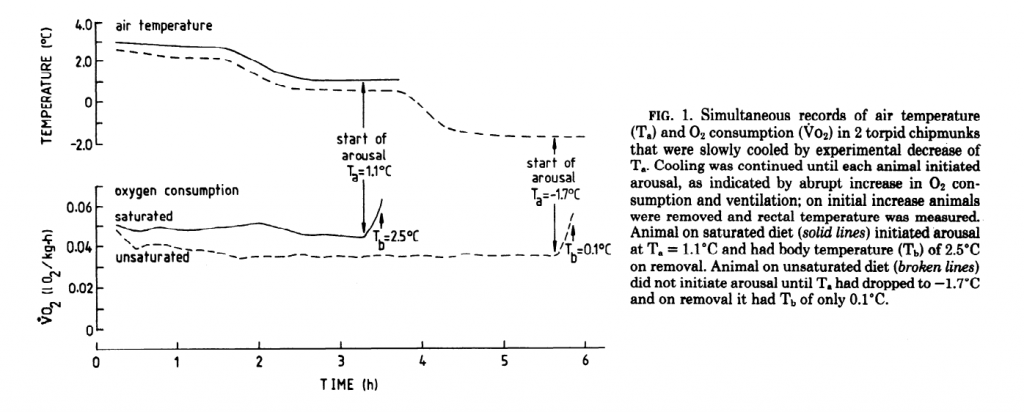
Certainly sheep tallow is just as rich in acetyl groups as is sunflower oil. What is the difference in their effect on torpor? The explanation will be in part II, which will be forthcoming this week. Enjoy til then!
- 1.Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity. Published online September 2010:1695-1700. doi:10.1038/oby.2009.510
- 2.Müllner E, Röhnisch HE, von Brömssen C, Moazzami AA. Metabolomics analysis reveals altered metabolites in lean compared with obese adolescents and additional metabolic shifts associated with hyperinsulinaemia and insulin resistance in obese adolescents: a cross-sectional study. Metabolomics. Published online January 2021. doi:10.1007/s11306-020-01759-y
- 3.Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Current Opinion in Cell Biology. Published online April 2015:125-131. doi:10.1016/j.ceb.2015.02.003
- 4.Chu LP, Swoap SJ. Oral bezafibrate induces daily torpor and FGF21 in mice in a PPAR alpha dependent manner. Journal of Thermal Biology. Published online July 2012:291-296. doi:10.1016/j.jtherbio.2011.11.011
- 5.Mathers KE, Staples JF. Differential posttranslational modification of mitochondrial enzymes corresponds with metabolic suppression during hibernation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. Published online August 1, 2019:R262-R269. doi:10.1152/ajpregu.00052.2019
- 6.Rouble AN, Storey KB. Characterization of the SIRT family of NAD+-dependent protein deacetylases in the context of a mammalian model of hibernation, the thirteen-lined ground squirrel. Cryobiology. Published online October 2015:334-343. doi:10.1016/j.cryobiol.2015.08.009
- 7.Geiser F, Kenagy GJ. Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. Published online May 1, 1987:R897-R901. doi:10.1152/ajpregu.1987.252.5.r897
- 1.Khamaisi M, Rudich A, Potashnik R, Tritschler HJ, Gutman A, Bashan N. Lipoic acid acutely induces hypoglycemia in fasting nondiabetic and diabetic rats. Metabolism. Published online April 1999:504-510. doi:10.1016/s0026-0495(99)90112-9

Are you implying that treating humans with fibrates which activates ppar alpha and keep them on their high n6 PUFAs diets they will potentially be torpid?
Well, this is tricky. I’m really talking about entrance to torpor here. Once one is torpid, PPAR alpha levels drop because they’re controlled by Sirt1. Once you are fully torpid, stimulating PPAR alpha might be a good idea.
Fatty acids that contain an odd number of carbon atoms are broken down in a via similar way to those that contain an even number. The only difference is the final product that is produced. In the case of even chain fatty acids, we generate acetyl CoA molecules. But in the case of odd chain fatty acids, we generate acetyl CoA as well as a propionyl CoA molecule. The propionyl CoA is ultimately transformed via a three-step process into succinyl CoA, which can then be incorporated into the citric acid cycle. This three step process involves three different enzymes – propionyl CoA carboxylate, methylmalonyl CoA racemase and methylmanlonyl CoA mutase. Note that the carboxylase requires biotin (vitamin B7) while the mutase requires vitamin B12 (deoxyadenosylcobalamin) for proper activity.
Good day Brad!!
I am one of your avid reader of your blog for almost 2 years now. I believed that suet fats (rich in stearic acid) provide efficient fuel in our mitochandria, and I am starting to eat rice again(I am diabetic since 2009) with suet fats from carabao and cow and feel well again. I started a facebook group here in the Philipines and I named the group CSE CARNIVORE FAT:PROTEIN RATIO and now have 628 members and I keep on posting your work their to keep members informed about your works, because I believed this will help members to avoid PUFA and will keep us healthy. Thank you Brad for keeping us informed.
Sorry for my slow comment moderation. People should check out this group! DO you have the link?
Yes, I have Brad I keep on posting your blog to our FB group.
Here is the link of the group https://www.facebook.com/groups/358121339047381/?ref=share_group_link