With every new paper that comes out it becomes clearer that reductive stress is the root cause of oxidative stress. I tried to explain this before, but I’m not sure how well I did.
Here’s how it works:
Some Initiating Event
There is an initiating event which pushes the cell into reductive stress, as defined by a high level of NADH. This could be vegetable oil consumption or environmental toxins such as forever chemicals in beef or drinking water which activate the Aryl hydrocarbon Receptor (AhR). Either way, the progression to oxidative stress starts with a small rise in the NADH/NAD+ ratio. This is the early stage of reductive stress.
The Cycle Continues
High NADH levels slow down the krebs cycle, resulting in an increase in acetyl-CoA. Acetyl-CoA comes from food (fat, carbs, alcohol) and is the fuel that acetylates mitochondrial enzymes and turns them off. ACETYLation requires ACETYL groups which are carried on ACETYL-CoA. Got it?
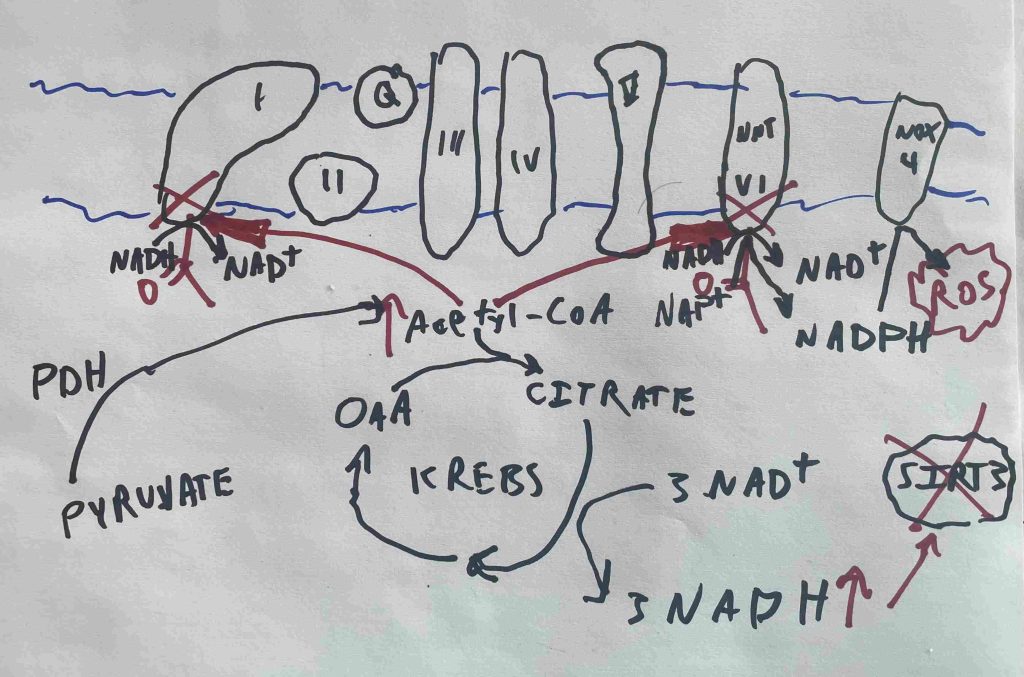
Sirtuin de-ACETYLases (Sirt3) remove the acetyl groups and turn the mitochondrial enzymes back on. High NADH levels inhibit the sirtuin deacetylases. High acetyl-CoA and NADH together lead to mitochondrial enzymes becoming acetylated and turned off.
In obese human children, complex I activity is low because it is acetylated:1
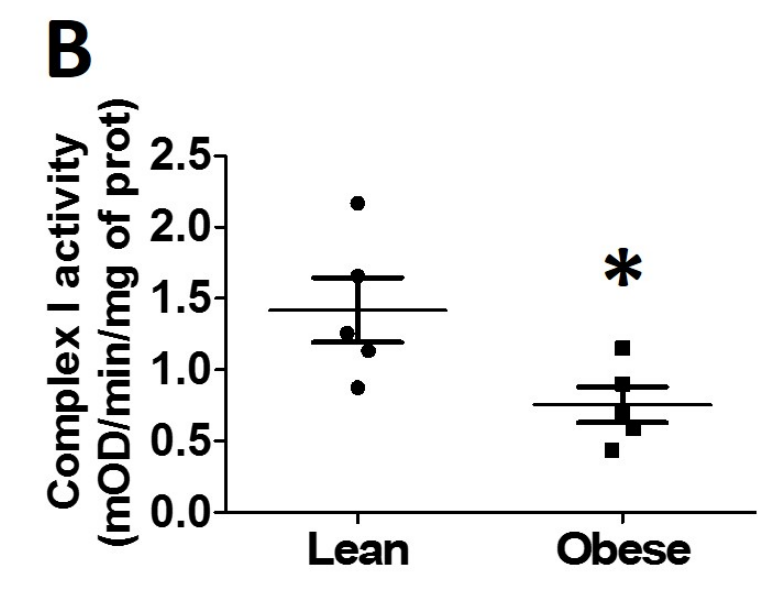
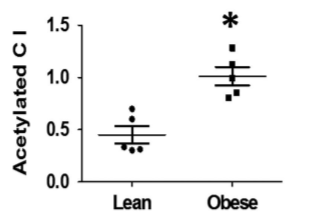
Acetylated Complex I Increases NADH/NAD+ Ratio
Complex I is also called NADH:Ubiquinone oxidoreductase. It is the main source in the cell where NADH is oxidized to NAD+ (NADH is oxidized, ubiquinone is reduced, hence “oxidoreductse”), restoring the NADH/NAD+ balance. If complex I activity is lowered, the NADH/NAD+ ratio rises2, enhancing reductive stress.
This is a positive feedback loop. High NADH levels lead to acetylated Complex I, which leads to high NADH levels.
High NADH/NAD+ Levels Increase ROS Production
I like the opening line of this abstract2: Reactive oxygen species (ROS) production by isolated complex I is steeply dependent on the NADH/NAD+ ratio. “Steeply dependent”.
The higher the level of NADH is, the more ROS will be produced at Complex I. NADH is an electron carrier. NADH is an antioxidant (via NNT/NADPH). Antioxidants are electron donors. Free radicals have unpaired electrons and donating an electron to them completes the pair and makes them less reactive.
Electron donors have the potential to generate ROS by donating the electron to oxygen instead, creating superoxide. Antioxidants are double edged swords. Vitamin C can be used to increase hydrogen peroxide levels to kill cancer cells.3,4
To be an antioxidant is to have the ability to generate ROS. High NADH generates a lot of ROS. Reductive stress causes oxidative stress.
High NADH Levels Lead to Low Reduced Glutathione Levels
The”master antioxidant” is glutathione. The enzyme Glutathione Peroxidase (GPx) uses reduced glutathione to eliminate hydrogen peroxide (H2O2), converting it to water (H2O). H2O2 is a reactive oxygen species (ROS) whose buildup leads to oxidative stress.
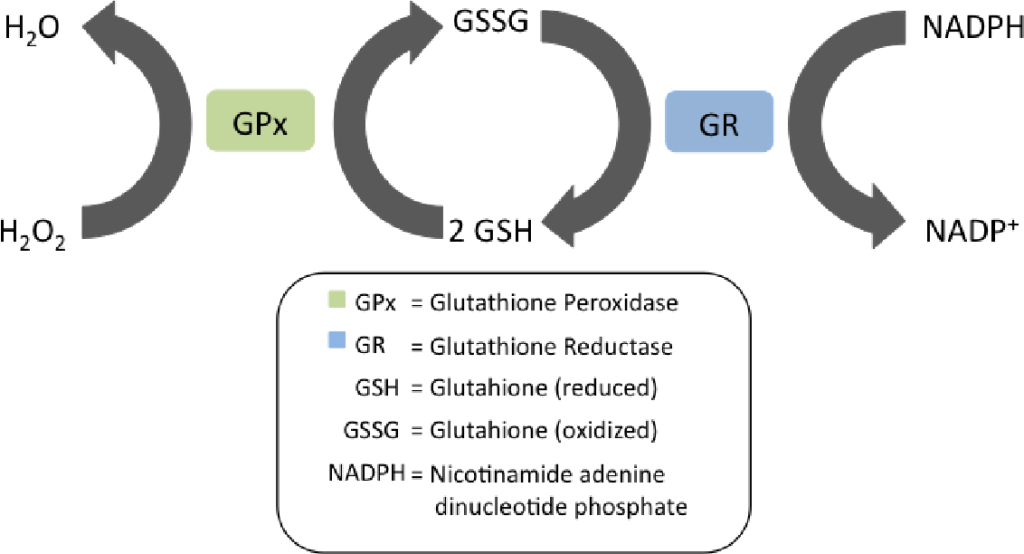
Every time a molecule of H2O2 is eliminated, a molecule of reduced glutathione becomes oxidized glutathione. An enzyme called Glutathione Reductuse (GR) converts oxidized glutathione back to reduced glutathione so that the reduced glutathione can eliminate another molecule of H2O2.
HERE’S THE KEY THING: Glutathione Reductase requires a molecule of NADPH to convert oxidized glutathione to reduced glutathione, which is necessary for the cell to eliminate ROS. The enzyme NNT (Complex VI) uses NADH to regenerate NADPH. In theory, high NADH levels should drive NADPH levels should drive ROS removal should drive normalization of the NADH/NAD+ ratio. In practice, NNT becomes acetylated5 when NADH and Acetyl-CoA levels are high.
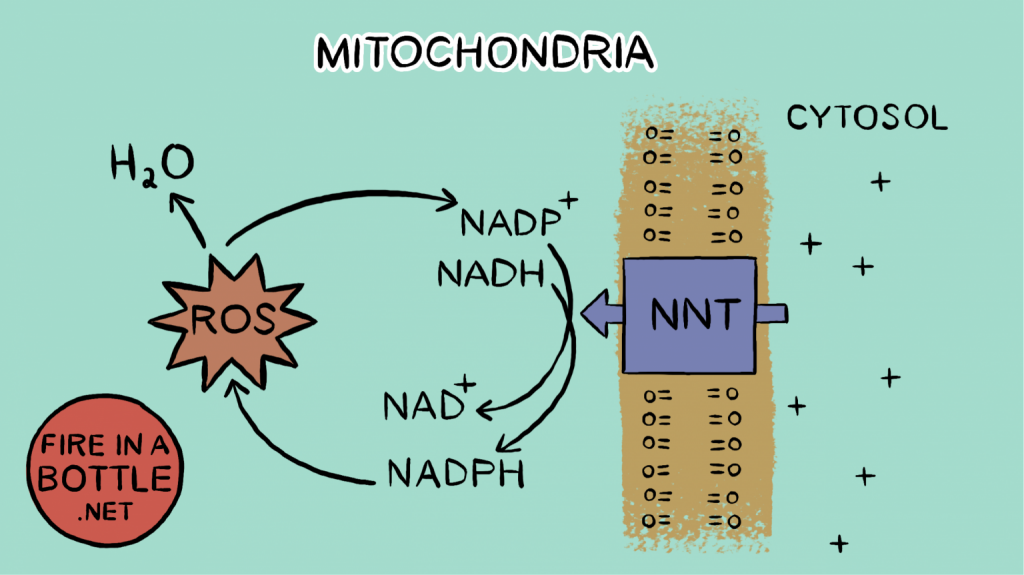
In practice, acetylated NNT leads to a drop in NADPH leads to high NADH levels leads to increased ROS production leads to decreased ability to remove H2O2 leads to a more oxidized glutathione pool leads to oxidative stress.
Pyruvate Eliminates Oxidiative Stress by Oxidizing NADH to NAD+
Eliminating oxidative stress takes oxidants. Pyruvate is an oxidant that circulates in the bloodstream and is easily taken into cells. Lactate dehydrogenase uses the oxidant power of pyruvate to oxidize NADH back to NAD+.
When cells are oxygen deprived – such as heart muscle during a heart attack – there is a huge buildup of NADH. Oxygen is the terminal electron acceptor and there is no where for the electrons held by NADH to go in the lack of it. There is not even any oxygen to make ROS.
When oxygen is reintroduced, there is a huge burst of ROS production due to the buildup of NADH. Because reductive stress causes oxidative stress. This leads to what is called ischemia-reperfusion injury. Ischemia is lack of oxygen and reperfusion is reintroducing oxygen.
If you take a guinea pig heart and withhold oxygen while the heart is fed pyruvate, you can reduce the amount of ROS generated when oxygen is reintroduced by 75%. In this way, pyruvate is said to be an “antioxidant”, but of course that is incorrect. It is an oxidant which reduces ROS production. Because reductive stress is the cause of oxidative stress. Pyruvate eliminates reductive stress.
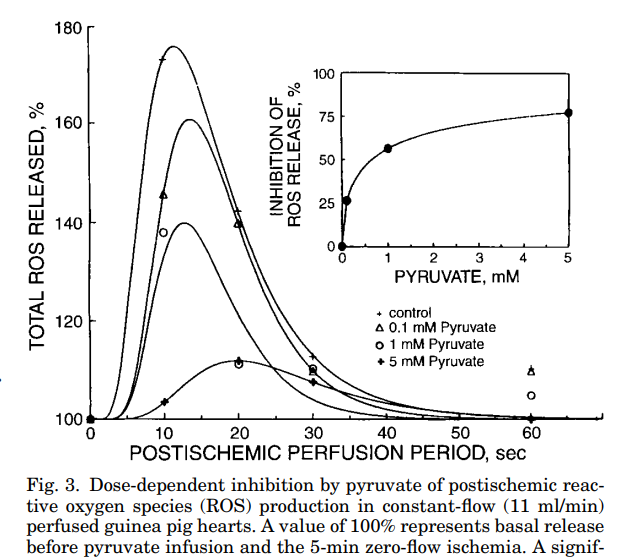
Conversely, you can feed the hearts lactate. Lactate is an antioxidant: lactate dehydrogenase uses it to eliminate the oxidized NAD+, reducing it to NADH. The reducing power of NADH drives our antioxidant systems through the NNT-NADPH-Glutathione Reductase pathway. The reducing power of NADH also creates ROS. Because antioxidants are the source of ROS.
When you feed the hearts lactate, ROS production is hugely increased.
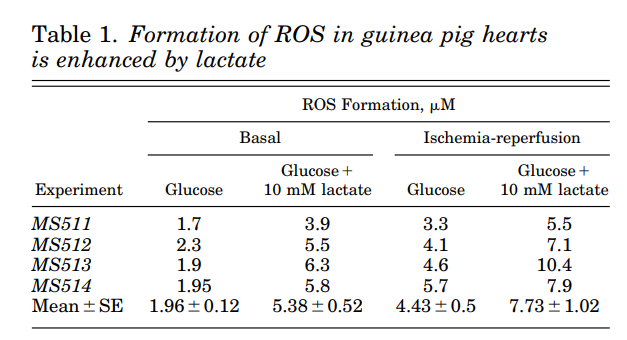
It’s also worth pointing out that even in the absence of ischemia-reperfusion, lactate dramatically increases ROS buildup. Lactate leads to an increase in NADH. Reactive oxygen species (ROS) production by isolated complex I is steeply dependent on the NADH/NAD+ ratio. “Steeply dependent.” Antioxidants are the source of ROS.
Using Pyruvate
I have been arguing in favor of calcium pyruvate as a weight loss supplement. You can save 12% on calcium pyruvate at bulksupplements.com if you use the promo code FIRE at checkout. I’ve stated that I think reductive stress is at the root of many of our metabolic problems and you can see here that pyruvate can be an extraordinary tool for dealing with oxidative stress. Just don’t call it an antioxidant. Antioxidants are electron donors and pyruvate is an electron acceptor.
In addition to oxidative stress, decreasing NADH levels with pyruvate will get Sirt1 and Sirt3 activated, leading to deactylation of mitochondrial enzymes. This will get you going! I recommend it with l-carnitine, which will pull acetyl-CoA groups from your mitochondria, multiplying the effect. Calcium pyruvate contains quite a bit of calcium, so you may want to consider a good vitamin K2 supplement as well. Vitamin K2 also helps to increase the NADH/NAD+ ratio!
I’ve been taking 12g of calcium pyruvate with 2g of l-carnitine – split over two servings – and vitamin k2. One level teaspoon of calcium pyruvate is 5-6g. I dissolve it with three teaspoons of sugar in hot water, chill it with ice and top it with seltzer. Pretty tasty. Bulk supplements also has a whole line of low calorie sweeteners.
Conclusions
Rising NADH levels lead to a buildup of acetyl-CoA and increase ROS production at mitochondrial Complex I. They also inhibit Sirt1 and Sirt3. High acetyl-CoA levels with low Sirt3 acitivity is a recipe for mitochondrial enzyme acetylation. Acetylated Complex I and acetylated NNT both push NADH levels even higher. Loss of NNT activity leads to a drop in NADPH levels which are required for ROS removal via glutathione.
Reductive stress, as defined by high levels of NADH and acetyl-CoA, drive oxidative stress as defined by high levels of ROS. Antioxidants – electron donors – are ultimately the source of ROS. It just depends on who they donate the electrons to.
Check back in. Later this week I’m hoping to build on this story. Reductive stress ALSO causes inflammation. Of course it does.
6
- 1.Zamora-Mendoza R, Rosas-Vargas H, Ramos-Cervantes MT, et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int J Obes. Published online November 21, 2017:618-624. doi:10.1038/ijo.2017.274
- 2.Korge P, Calmettes G, Weiss JN. Reactive oxygen species production in cardiac mitochondria after complex I inhibition: Modulation by substrate-dependent regulation of the NADH/NAD+ ratio. Free Radical Biology and Medicine. Published online July 2016:22-33. doi:10.1016/j.freeradbiomed.2016.04.002
- 3.Wu CW, Liu HC, Yu YL, Hung YT, Wei CW, Yiang GT. Combined treatment with vitamin C and methotrexate inhibits triple-negative breast cancer cell growth by increasing H2O2 accumulation and activating caspase-3 and p38 pathways. Oncology Reports. Published online February 10, 2017:2177-2184. doi:10.3892/or.2017.5439
- 4.Bonilla-Porras AR, Jimenez-Del-Rio M, Velez-Pardo C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signalling mechanism. Cancer Cell Int. Published online June 10, 2011. doi:10.1186/1475-2867-11-19
- 5.Luo C, Ding W, Zhu S, Chen Y, Liu X, Deng H. Nicotinamide Mononucleotide Administration Amends Protein Acetylome of Aged Mouse Liver. Cells. Published online May 16, 2022:1654. doi:10.3390/cells11101654
- 6.Schreck R, Albermann K, Baeuerle PA. Nuclear Factor Kb: An Oxidative Stress-Responsive Transcription Factor of Eukaryotic Cells (A Review). Free Radical Research Communications. Published online January 1992:221-237. doi:10.3109/10715769209079515

Thanks for explaining things for those of us not so biochemically inclined! At this point are you thinking that the pyruvate and l-carnitine might eventually be taken in smaller doses as an individual improves his/her metabolic health? Might we be able to eliminate both supplements when/if we restore our metabolic health?
I am always hoping to avoid long term or even lifelong supplement needs, but can see a benefit over shorter time frames as a means to assist healing. I’m curious to hear your ongoing results with this protocol!
Hi Brad,
You mention taking K2 which makes sense to me but what about the D3 to go with it to help send the calcium where you want it etc
Or am I missing something and the D3 is a bad thing when taking this level of calcium ???
Not Brad but I’ve been reading and can chime in with what I know.
K2 makes sure calcium goes to your bones and not forming osteoclasts in your arteries – saw a youtube on this and the blood vessels tend to calcify which K2 opposes.
D3 mainly enables your gut to absorb calcium from your diet instead of robbing your bones. It does a lot of other stuff and maybe interacts with K2 but it’s obvious why they work together.
Extra dietary calcium should help D3 do its job with less D3 required but if you have lots of calcium in the blood, from what I’m gathering so far, you BETTER have K2 there to keep it from going to the arteries! That’s my current (prolly incomplete) understanding right now.
There is a whole nother sub-hypothesis for how heart disease develops that seems to start with the Western diet eschewing traditional fermented foods and thus folks getting chronically deficient in K2. I wonder where the science will end up going on that front.
Thanks Eric, really appreciate your thoughts on this and that does make perfect sense.
I find the calcium pyruvate unpalatable- tried the seltzer trick, coffee, chocolate shake. It ruins all of them. The Succinate is pretty bad but I’ve gotten used to it.
Any recipes for making this taste better? Otherwise I’ll have 950 grams to give away…
I found the trick with the calcium pyruvate is to add more sweetener to balance the acidity. I’ve been using three teaspoons of sugar with a teaspoon of calcium pyruvate and it’s really pretty tasty. I suspect other sweeteners would work the same if you don’t want the sugar.
Brad
One of beet root powder mechanisms is upregulating PDH. Oxphos increased. Also BR has alpha lipoic acid.and flavonoids to counter CD38.
https://www.frontiersin.org/articles/10.3389/fnut.2021.660150/full
Forgot paper
Yeah… Beet root has nitrate, which is converted to nitric oxide. Which is removed by glutathione. WHich oxidizes NADPH, which in turn oxidizes NADH back to NAD+. Interesting.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6836164/
Interesting. Of course upregulated PDH implies decreasing reductive stress…
I buy from bulk supplement store. But no allulose for sweetener. See Peter Attia for blog entry on allulose. Much better option imo.
is it better to take pyruvate or succinate? Don’t they play the same role as parts of the Krebs cycle?
They play similar but different roles. The pyruvate is allowing lactate dehydrogenase in the cytolplasm to offload some NADH. The succinate is helping to drive some extra ROS in the mitochondria – especially when burning extra unsaturated fat which has low succinate dehydrogenase input. Those ROS also help us to ultimately get rid of NADH via the glutathione-reductase/NNT pathway, but for the succinate to be fully effective, it is important to FIRST get succinate dehydrogenase (complex II) deactylated. The purpose of pyruvate/l-carnitine is deacetylation.
They should all complement each other, but maybe START with the pyruvate/l-carnitine.
Brad
does succinate perform the same function as pyruvate?
They play similar but different roles. The pyruvate is allowing lactate dehydrogenase in the cytolplasm to offload some NADH. The succinate is helping to drive some extra ROS in the mitochondria – especially when burning extra unsaturated fat which has low succinate dehydrogenase input. Those ROS also help us to ultimately get rid of NADH via the glutathione-reductase/NNT pathway, but for the succinate to be fully effective, it is important to FIRST get succinate dehydrogenase (complex II) deactylated. The purpose of pyruvate/l-carnitine is deacetylation.
They should all complement each other, but maybe START with the pyruvate/l-carnitine.
Brad