Since I published The Croissant Diet there has been much interest in the scientific article I call “The banana milkshake study“1. The study showed that ingestion of 24g of stearic acid in a banana shake after a two day low fat diet caused mitochondrial fusion and a drop in circulating acylcarnitines.
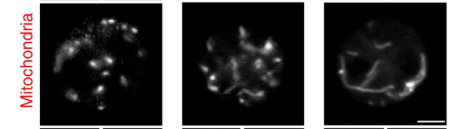
We are very interested in this since mice fed stearic acid have shredded abs.2
The authors of the banana milkshake study argue that the mitochondrial fusion is due to stearoylation of an enzyme – TfR1 – that is involved in signalling pathways that effect mitochondrial fusion. I suspect that this is true, but I also suspect that the mitochondrial fusion is happening due to stearic acid’s effect on reductive stress – freeing up NAD+. As I like to say, these things are typically regulated by multiple reinforcing feedback loops. Turtles all the way down.
In recent posts I have argued that obesity is caused by reductive stress. One way to cause reductive stress is by shuttling fat, especially unsaturated ones, into the mitochondria more quickly than NAD+ can be regenerated, leading to a high ratio of NADH to NAD+ (NADH is the REDUCED form, NAD+ is oxidized). NAD+ is the limiting factor in fat burning and when you are short on it, you get a buildup of NADH, acetyl-CoA and circulating acylcarnitines. Not good!
How does stearic acid battle reductive stress?
The vast majority of dietary fats we consume are composed of just four fatty acids: the saturated stearic and palmitic acids, the monounsaturated oleic acid and the polyunsaturated linoleic acid. Three of these – stearic, oleic and linoleic – are 18-carbon fats that vary only in degree of unsaturation. Of these three, stearic acid is by far the most slowly absorbed and oxidized. Consider the percentage of the three fats that is burned over the first 9 hours after ingestion3:
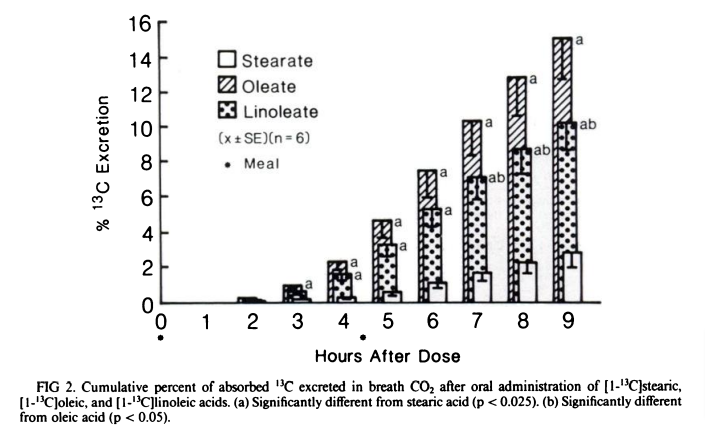
Dietary stearic acid is bareley even oxidized (burned in the mitochondria). In fact, stearic acid is the slowest to be burned at every level. When you eat fat, it is packed into chylomicrons by the intestine, from which it s released as free fatty acids which are taken up by cells through the transporter CD36 and ultimately transferred into the mitochondria by an enzyme called CPT1a, whose expression is controlled by PPAR alpha.
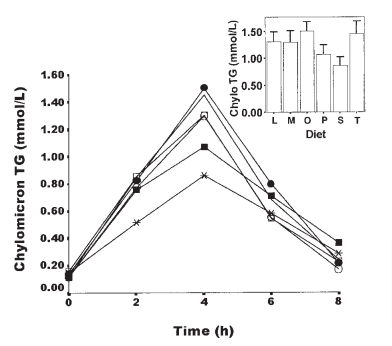
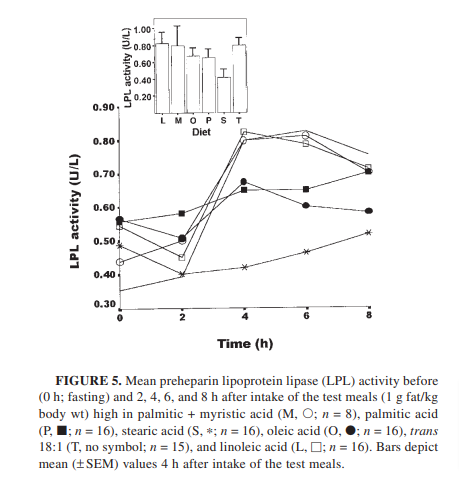
Step 1, absorption: Stearic acid leads to low rates of chylomicron formation after a meal.4 The stearic acid rich blend is the line with the x and S in the chart. The other letters in the chart are blends high in linoleic acid, myristic acid, oleic acid, palmitic acid and transfat.
Step 2, release from chylomicrons as free fatty acids (FFA) : Chylomicrons “park” at tissues where the fats inside are released into the bloodstream as FFA. The enzyme that releases them is called lipoprotein lipase. LPL has low activity on stearic acid compared to other fats.4
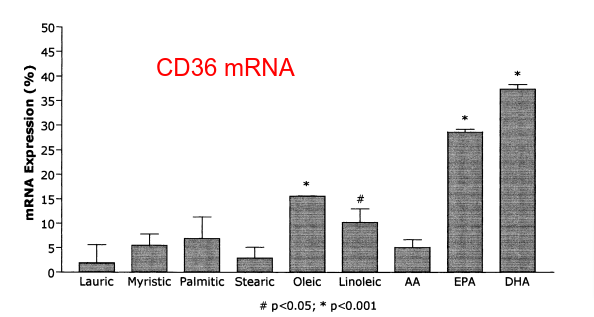
Step 3, uptake into cells: The enzyme CD36 is involved in free fatty acid uptake into cells. Its expression is under the control of PPAR gamma, which is activated by oleic and linoleic acid but NOT by stearic acid. Stearic acid leads to low levels of CD36.5
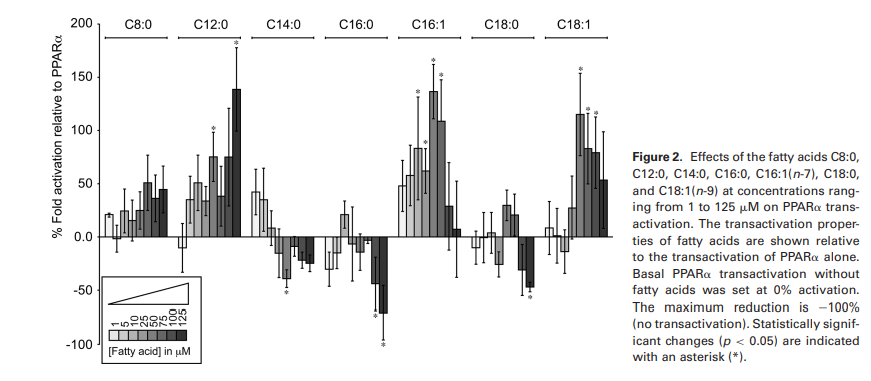
Step 4, entry into the mitochondria: The enzyme that controla the rate that fat enters the mitochondria is called CPT1, whose expression is controlled by PPAR alpha. PPAR alpha is stimuluted by oleic acid (C18:1) but not stearic (C18:0).6
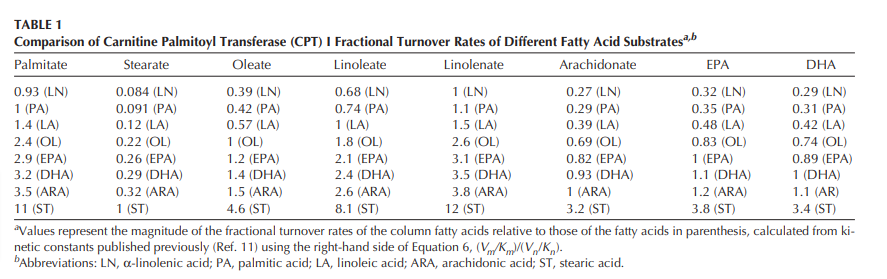
Step 4a, entry into the mitochondria: PPAR alpha controls the AMOUNT of CPT1, but the enzyme itself also has different preferences for which fats it prefers to shuttle into the mitochondria. Stearic acid is dramatically dis-preferred by CPT1.7
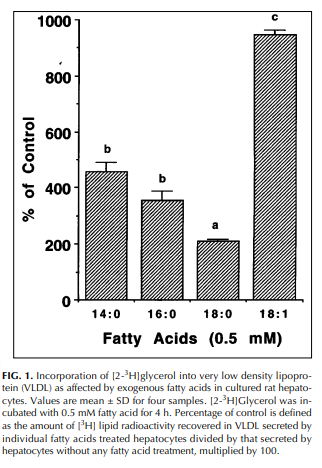
Step 5, re-release: FFA that are not absorbed by tissues are taken up by the liver, made back into triglycerides and released as VLDL, which behaves much like chylomicrons. Stearic acid is poorly incorporated into VLDL.8
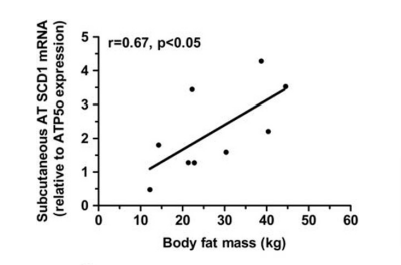
At literally every step in the pathway of fat oxidation, stearic acid is the slowpoke. Stearic acid is putting the brakes on the flood of fat into mitochondria to allow them to keep up. In contrast, oleic acid – which stearic acid is rapidly converted to by the enzyme SCD1 – is a racehorse. Is it any wonder that hibernating animals ramp up SCD1 expression to help them get torpid?9 Or that there is a direct correlation in humans between SCD1 expression in adipose tissue and fat mass?10
I recently wrote about how large doses of linoleic acid – another racehorse – leads to increased fat oxidation in the short term, which limits your ability to burn glucose, just like in human obesity. A metabolic hallmark of type II diabetes is actually the inability to lower FFAs (aka NEFA:non-esterified fatty acids) following a mixed meal of carbohydrate and fat coupled with high postprandial fat oxidation and low glucose oxidation.11
And consider these facts: the natural SCD1 inhibittor sterculia oil12, which blocks conversion of stearic acid to oleic acid, improves glycemic control in a spontaneously obese strain of rats. Mice given a pharmeceutical that lowers lipase activity13 and therefore levels of FFAs, have excellent glycemic control.
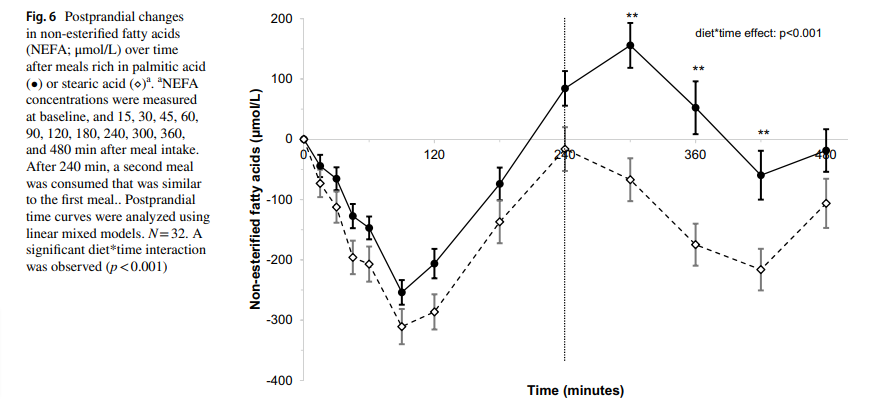
What does all of this slowing down of fat flow by stearic acid add up to? A 2021 paper showed the effects of stearic acid on postprandial FFA levels.14 One of the main jobs of insulin is to suppress lipolysis to lower postprandial FFA levels. This prioritizes the burning of glucose to prevent blood glucose spikes. If you are eating a mixed diet, you WANT to suppress FFA after a meal. Compared to palmitic acid, stearic acid dramatically lowers postprandial FFA, especially after consecutive meals.
I’ve already mentioned that vegetable acutely lowers glucose oxidation, just like in diabetics. Stearic acid appears to be singularly good at allowing for post-prandial glucose oxidation. Stearic acid causes you to behave like a lean person.
Eliminating reductive stress causes mitochondrial fusion.
OK, but so then why does stearic acid cause mitochondrial fusion, decreased circulating acylcarnitines and “fat burning mode” if it actually SLOWS DOWN the rate of fat oxidation? Is this a paradox?
Do you want to be the tortoise or do you want to be the hare?
Here’s my take on the banana milkshake study. Since most Americans have high levels of stored oleic and linoleic acids, when they went on a low fat vegan diet for two days, the participants probably experienced reductive stress. Low NAD+ levels are associated with mitochondrial fragmentation.15 When they consumed the banana shake, it led to a slowdown in fat processing, allowing for the mitochondrial recovery of NAD+ levels, which caused the fusion. In addition, SOME of the stearic acid was burned and saturated fat produce more NAD+ via the ROS/GR/NNT pathway. Mitochondria that are fused have larger volume and surface area, their mitochondrial enzymes are not acetylated and they are able to process fat more efficiently, leading ultimately to the reduction of circulating acylcarnitines. Slwoing down the rate at which fat enetered the mitochondria after a mixed meal restored NAD+ levels of the participants, putting them into fat burning mode.
Conclusion
There are a lot of lines of evidence suggesting that stearic acid’s effects on mitochondrial fusion and fighting abdominal fat have as much to do with the dynamics of how it gets into the mitochondria as anything else. Stearic acid is the tortoise of fats. Slow and steady wins the race.
Reducing postprandial FFAs can play a huge role in restoring glycemic control since fat oxidation blocks glucose oxidation via the randle cycle. You wouldn’t be too wrong to think of stearic acid as “the fiber of fat”.
- 1.Senyilmaz-Tiebe D, Pfaff DH, Virtue S, et al. Dietary stearic acid regulates mitochondria in vivo in humans. Nat Commun. Published online August 7, 2018. doi:10.1038/s41467-018-05614-6
- 2.Shen MC, Zhao X, Siegal GP, Desmond R, Hardy RW. Dietary Stearic Acid Leads to a Reduction of Visceral Adipose Tissue in Athymic Nude Mice. Weiss SFT, ed. PLoS ONE. Published online September 15, 2014:e104083. doi:10.1371/journal.pone.0104083
- 3.Jones PJH, Pencharz PB, Clandinin MT. Whole body oxidation of dietary fatty acids: implications for energy utilization. The American Journal of Clinical Nutrition. Published online November 1, 1985:769-777. doi:10.1093/ajcn/42.5.769
- 4.Tholstrup T, Sandström B, Bysted A, Hølmer G. Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. The American Journal of Clinical Nutrition. Published online February 1, 2001:198-208. doi:10.1093/ajcn/73.2.198
- 5.Vallvé JC, Uliaque K, Girona J, et al. Unsaturated fatty acids and their oxidation products stimulate CD36 gene expression in human macrophages. Atherosclerosis. Published online September 2002:45-56. doi:10.1016/s0021-9150(02)00046-1
- 6.Popeijus HE, van Otterdijk SD, van der Krieken SE, et al. Fatty acid chain length and saturation influences PPARα transcriptional activation and repression in HepG2 cells. Mol Nutr Food Res. Published online October 27, 2014:2342-2349. doi:10.1002/mnfr.201400314
- 7.Gavino VC, Cordeau S, Gavino G. Kinetic analysis of the selectivity of acylcarnitine synthesis in rat mitochondria. Lipids. Published online April 2003:485-490. doi:10.1007/s11745-003-1088-7
- 8.Pai T, Yeh YY. Stearic acid modifies very low density lipoprotein lipid composition and particle size differently from shorter-chain saturated fatty acids in cultured rat hepatocytes. Lipids. Published online February 1997:143-149. doi:10.1007/s11745-997-0018-z
- 9.Chayama Y, Ando L, Sato Y, et al. Molecular Basis of White Adipose Tissue Remodeling That Precedes and Coincides With Hibernation in the Syrian Hamster, a Food-Storing Hibernator. Front Physiol. Published online January 28, 2019. doi:10.3389/fphys.2018.01973
- 10.Caron-Jobin M, Mauvoisin D, Michaud A, et al. Stearic acid content of abdominal adipose tissues in obese women. Nutr & Diabetes. Published online January 2012:e23-e23. doi:10.1038/nutd.2011.19
- 11.Normand-Lauzière F, Frisch F, Labbé SM, et al. Increased Postprandial Nonesterified Fatty Acid Appearance and Oxidation in Type 2 Diabetes Is Not Fully Established in Offspring of Diabetic Subjects. Vella A, ed. PLoS ONE. Published online June 4, 2010:e10956. doi:10.1371/journal.pone.0010956
- 12.Ortinau LC, Nickelson KJ, Stromsdorfer KL, et al. Sterculic Oil, a natural inhibitor of SCD1, improves the metabolic state of obese OLETF rats. Obesity. Published online February 2013:344-352. doi:10.1002/oby.20040
- 13.Schweiger M, Romauch M, Schreiber R, et al. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun. Published online March 22, 2017. doi:10.1038/ncomms14859
- 14.van Rooijen MA, Plat J, Zock PL, Blom WAM, Mensink RP. Effects of two consecutive mixed meals high in palmitic acid or stearic acid on 8-h postprandial lipemia and glycemia in healthy-weight and overweight men and postmenopausal women: a randomized controlled trial. Eur J Nutr. Published online March 17, 2021:3659-3667. doi:10.1007/s00394-021-02530-2
- 15.Klimova N, Fearnow A, Long A, Kristian T. NAD+ precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Experimental Neurology. Published online March 2020:113144. doi:10.1016/j.expneurol.2019.113144

Why not supplement NAD+?
I have no problem with supplementing NAD+ precursors such as NR and NMN. I’m sure they’re helpful but they’re also not the full story.
I’ve been thinking about this. Alpha lipoic acid oxidizes NADH to NAD+ thus improving the NAD/NADH ratio. Niacinamide (a form of vitamin B3) supplementation raises NAD+ levels. Combining both seems to me as the best strategy to combat reductive stress.
Also it is not clear to me – is it because of higher NAD+ that we see mitochondrial improvements with stearic acid and SCD1 suppression with ALA? Also what about the FAD/FADH ratio? Will supplementing with riboflavin (vitamin B2) have metabolic benefits because it raises FAD? Those are questions that I would love to hear Brad answer since taking vitamin B2, B3 and ALA is easy and inexpensive.
Hi Brad, how much stearic acid do you recommend to take daily?
I think it’s best used with or right before meals. 24g was enough to create mitochondrial fusion. I’ve been taking more like 40g.
Great post, getting more texture around the problem. So the general strategy which you said you’ll outline is starting to look to me like:
1. Provide an antidote to the excessive CD36/CPT1 at all times of day, or if you can’t, recycle NADH back into NAD+ forcibly by some agent
2. During feeding times, we can use stearic acid and sterculia oil to help improve the FADH2/NADH ratio to improve upon this
3. Succinate will also help drive the ROS/GR/NNT pathway during feeding times (? only feeding times or also between feeding)
4. Do we just look to Alpha Lipoic Acid (and/or succinate?) as an antidote to the reductive stress during non-feeding (i.e. when we’re metabolizing all that oleic acid our fatazz has stored)?
Yup, we’re coming around to it. Of course, I don’t have all of the answers, but getting a better understanding of the problem is of course the first step.
Brad
Hi Brad!
Just wanted you to know that I’m FINALLY losing weight!!!
I just gave up every speck of processed food in my life lol i shouldn’t say ‘just’ coz i’m not saying that it would be an easy path for everyone out there lol
I just applied your knowledge to whole foods and I’m fine now! Just wanted you to know…
I didn’t cut out anything, except the poisons… i did NOT decide to go low fat immediately or anything, I still eat how you suggest, it’s just that whatever they put in processed food was keeping me fat.
anyway… i know i wrote to you with a lot of struggles, but i was missing the one point that was key for me
and in fact i HAD eaten whole foods in the past, but that’s before i met you lol… i needed your knowledge 🙂
thanks, Brad xox
Wow, Elaine!
This is great news!! Congratulations! I wish I could put emogee on here!
Do you mind sharing what your diet is like that has been working?
Hey Brad, love your work. I’d be lying if I said I understood half of the biochemistry you discuss in your articles. I went keto and experienced massive fat loss (30lbs in 6-8months) which led me to carnivore. However, I’m finding the stomach tire is coming back and, now with this article on stearic acid, I’m as confused as ever. Is low-carb not the root cause lever to pull for fat loss? Do we all just need MORE saturated fat in our diets?
I think that’s probably too simple. Keto/carnivore is a great dodge if you are failing to burn glucose efficiently. It doesn’t always work forever. Most muscle meats are not that high in stearic acid, anyway. I’m trying to focus on the underlying biochemical problems. In any individual, different dietary approaches may effect the underlying biochemistry differently depending on levels of reductive stress/SCD1, etc.
My personal goal is to become an efficient glucose burner again, but we’ll see if I can pull it off.
Brad
What do you think about combining sugar with stearic acid? I’m imagining it could cause a decent boost in metabolism based on your work and Ray Peat’s; c18 for mitochondrial fusion (bigger engine), sugar as the race octane.
I’m still more of a believer in starch than sugar. I think this is due to the fact that sugar upregulates SCD1.
Could niacinamide/b3 have the same effect?
Niacinimide won’t slow fat dynamics, but it may give you an NAD+ boost.
Do you think that palmitic acid does this as well?
Much less so.
Hi there — it seems that pure stearic acid is good to take to achieve these results but I would like to confirm that I don’t need to convert the stearic acid to butteroil. Is that the case?
No, it doesn’t need to be in butteroil. That was just a convenient way for me to consume it.
If you are low carb/keto/carnivore, could this slow processing of the stearic acid make you feel slow and less energetic after a meal consisting of very few carbs and a relatively high percentage of the fats beeing stearic acid? I mean if your mitochondria can’t get the fuel it needs quickly enough because of the slow absorption of the stearic acid?
That’s unclear. I think for MANY people, the fat consumption triggers satiation and the slow transport allows you to work through a backlog of energy as NADH/Acetyl-CoA. That gets your mitochondrial engine firing and allows you to generate more ATP. For those who are very lean/hypermetabolic, I might expect the opposite reaction. I’m just guessing.
Brad
I’ve had good results with bakers chocolate, plus coco butter, plus stearic acid powder, plus butter, plus cream and sweetener of choice. Ultimate stearic acid bombs! Talk about energy boost…..
Let me know if I understand the article correctly: so for a typical American with a high percentage of their own adipose tissue having PUFA, would it be best/protective for their mitochondria if they ate stearic acid with their carbs, instead of just eating carbs separately (aka thinking of the Rande cycle)? Because if they go on a low/no fat, normal protein diet, they’ll start to burn their own fat as needed, which happens to be higher in PUFA so their mitochondria would suffer? But in that case, I don’t understand how a person can lose their stored fat.
Yup, you’ve hit on the essential paradox and I’m not sure anyone knows the answer. I’ve been trying a lower fat diet, but the fat that I DO use is very high in stearic acid. My goal is to become an efficient carb burner again. The reason I think it’s good to burn carbs is basically because that’s what lean people do. Also, burning carbs results in less reductive stress than burning fat.
Here’s the good news: you are ALWAYS burning SOME fat, it’s never zero, it’s just a question of proportion.
Brad
If someone in Europe is interested – I managed to get my hands on some of the stearic acid that was used in the trial: food grade, 99% purity [classed at >95% purity but the analysis for my batch is 99%], expiry 2024, no rancid smell or taste, no fatty film in the mouth when eating after it has been melted, washes clean easily without leaving a film on anything, single source from palm oil, absolutely great. It wasn’t easy to get but I am glad I did it. I can spare a little since I found out that I don’t need that much.
Yes, I make the shakes, but only 2 or 3 times a week as a first meal of the DAY and with about 15grams of the SA. I find that I am not digesting all of it if I use more, so why waste it. Very energizing and satiating. I sometimes add a teaspoon to my first meal on other days but not consistently, about 5 grams. It has no taste so I don’t really notice it even if it’s not melted into the milk. Good for the girth too. I can eat higher carb now and am shedding weight. Avoiding seed oils like the plague too. It works. Plus I am feeling better. So thanks Brad for putting this out there :o).
Anyways, if Brad is so inclined to let me post this, if you are in Europe, better if you are in Germany and want some mailed [or are in Berlin and want to pick up], there is a cost of course, contact me at optin [at] wolke7.net. I can spare about 3KG in total since I can order again in the new year, but I can only order a limited amount once a year. Will only mail within Europe. So maybe two people with 1.5KG each… that will last you a while.
Btw, if anyone is making the shakes, don’t use homogenized milk since it won’t emulsify the SA when heated and stay separated.