This is the next in a series of posts I am writing about reductive stress as defined by the NAD+/NADH. This post looks at the uniqueness of superoxide as a reduced electron carrier. Superoxide is not like the other electron carriers. Most electron carriers, such as NADH, need to give their electron to something else before they can accept another. When oxygen gets an electron to become superoxide, it takes two more!! This is the key to escaping reductive stress.
For background, check out Meet your Antioxidant System, Superoxide: An Unlikely Hero, ROS as a NAD+ Regenerating Thermogenic Cycle, and Obesity as a Global Succinate Dehydrogenase Activity Deficit.
Reductive Stress
Redox biology describes the flow of electrons. Electrons flow from the carbon and hydrogen in fats and carbohydrates to molecular Oxygen – O2. O2 is the “terminal electron acceptor”. The electrons don’t flow along wires, they flow through electron carriers, such as NAD+. When NAD+ absorbs an electron (and a proton, H+, which are readily available in solution), it becomes NADH.
Things that gain electrons are reduced and things that lose electrons are oxidized. You can remember this with the mnemonic OILRIG, which means Oxidation Is Losing, Reduction Is Gaining with respect to electrons.
NADH is the reduced form of NAD and NAD+ is the oxidized form. Fat, glucose and ethanol are all converted to acetyl-CoA before entering the citric acid cycle. Each turn of the citric acid cycle requires three oxidized NAD molecules – NAD+. The less NAD+ is within the mitochondria, the slower the citric acid cycle can proceed and the slower your metabolism can run.
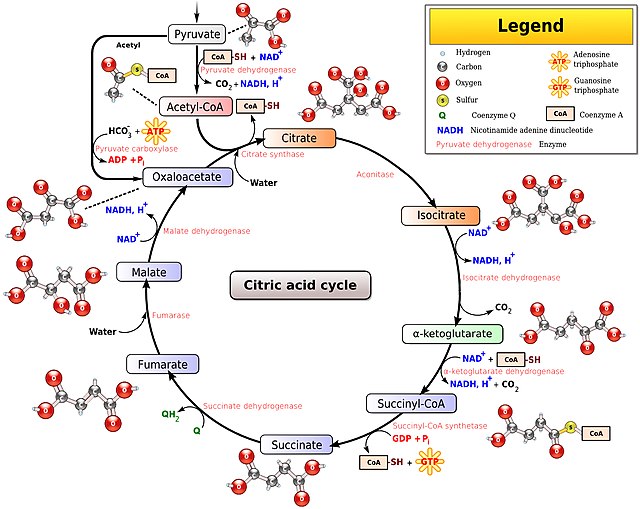
Mitochondria with a relatively high ratio of the reduced NADH to the oxidized NAD+ are said to have “reductive stress”. Metabolism cannot proceed efficiently because there are not enough available electron carriers.
The classic way that NADH becomes oxidized back to NAD+ is by handing it’s electrons to Complex 1 of the mitochondrial electron transport chain. Whenever a redox reaction happens between two molecules, one is reduced and the other is oxidized. So in this reaction Complex 1 is reduced. Remember that Reduction Is Gaining and it gained the electron that NADH lost. Oxidation Is Losing.
Biology has a bunch of “redox couples”, which is what NAD+ and NADH are collectively called. Others include FAD and FADH2; glutathione, which cycles between oxidized (GSSH) and reduced (GSH); and lipoic acid, which cycles back and forth between lipoic acid (oxidized) and dihydrolipoic acid (reduced). The only way for one of these that is reduced to become oxidized is for it to reduce something else. You have to have someone to hand the electron to.
Why do cells get reductive stress?
The electron transport chain works by pumping protons across the inner membrane to create a voltage gradient. The protons flow back through Complex V, releasing energy that is used to convert ADP to ATP. When energy levels are high – which can be from high blood glucose after a meal or high levels of free fatty acids while fasting; when energy demand is low – perusing Facebook or reading this blog; the ADP becomes converted to ATP, slowing the flow of protons through Complex V. The voltage gradient across the membrane becomes higher. It becomes harder for NADH to hand it’s electrons to Complex I, which has to push harder to pump protons. NADH levels build up and NAD+ levels drop.
At this point acetyl-CoA levels begin to climb, starting a feedback loop that slows down fat oxidation by inhibiting CPT1, the rate limiting enzyme in beta-oxidation.
This is reductive stress and the outcome is fat buildup.
Superoxide
Let’s say that you’re from a starch eating culture. You have very saturated body fat. It’s morning time and you are starting to make breakfast. It’s harvest season, food is plentiful, you’ve been feasting and packed on a few lbs. Your leptin levels have increased and so AMPK is phosphorylated and your body fat is being rapidly oxidized. Energy demands are low since you’re only casually getting breakfast together. Do you rapidly hit a state of reductive stress since free fatty acids are plentiful, AMPK is activated (fat is being burned quickly) and activity levels are low? Where will the electrons flow to? What is the source of NAD+?
The reduced electron carriers – NADH and FADH2 – have to push electrons onto the electron transport chain. If ATP levels are high and the proton gradient is high this process will proceed slowly. This is the very situation in which superoxide will be maximally generated – low insulin (you’re fasted), high proton gradient, activated AMPK, saturated fat.
When the proton gradient is high and saturated fat is being oxidized quickly, succinate dehydrogenase activity is maximal. Succinate dehydrogenase is the prime driver of mitochondrial ROS production. The electrons entering the chain through succinate dehydrogenase move backwards through Complex I and “ping” out through a process called reverse electron transport (RET). These electrons form superoxide.
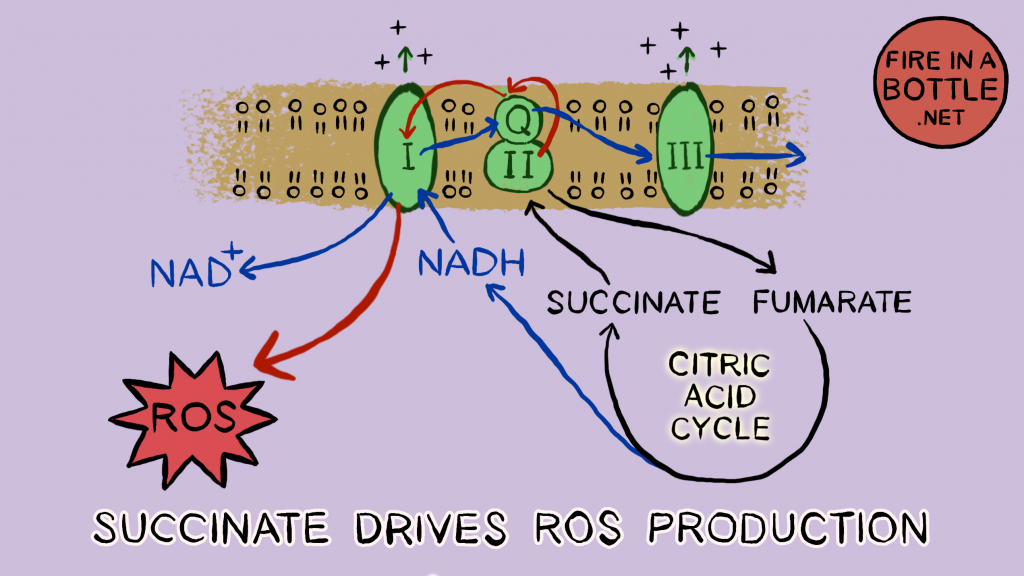
Superoxide is a reduced electron carrier. It is O2–, an oxygen that has absorbed an extra electron. Reduction Is Gaining (electrons). Who does it hand its electron to?
Superoxide Oxidizes Things
When I was a kid, in the early 80s, my parents surprised us one Christmas with a cable TV subscription and a Betamax recorder. I recorded Superman II off of HBO. In Superman II, Superman decides to marry Lois Lane. A holographic image of his mother tells him that he has to relinquish his super powers to marry a human woman. He goes to the far North and enters the Crystal Chamber, which takes his powers away. Later bad guys come from his birth planet and he ventures North to retrieve his powers to fight them, but there are three of them and only one of him. They hear of the Crystal Chamber, imprison Lois Lane and the whole party moves to the frozen North where Superman is forced into the Crystal Chamber and….. TAKES AWAY THE POWER OF THE OTHER THREE SUPERPEOPLE WHO ARE OUTSIDE OF THE CRYSTAL CHAMBER!!!
It was the ultimate switcheroo.
The enzyme superoxide dismutase (SOD2) rapidly converts two molecules of superoxide into hydrogen peroxide (H2O2) and O2. It’s worth taking a look for a second at how this happens. Superoxide Dismutase has a manganese ion at its core. Manganese is a metal and metals are very flexible from a redox perspective. This is why electrons flow through a copper wire. When SOD2 makes contact with a superoxide it transfers the extra electron onto its manganese, oxidizing the superoxide back to oxygen.
When the now-reduced SOD2 (Reduction Is Gaining) makes contact with a second superoxide, it transfers the extra electron from its manganese to the superoxide, reducing it again! This would make an oxygen with a charge of 2-, having 2 extra electrons except that a couple of protons are grabbed out of solution to make H2O2 instead.
THE SUPEROXIDE IS REDUCED A SECOND TIME! Reduction Is Gaining. This is the magic. SOD gives the extra electron to no one. Oxygen is an electron taker and the formation of superoxide starts the process.1
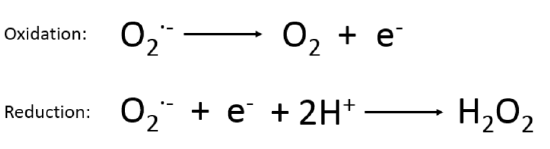
In the next reaction, glutathione diffuses the hydrogen peroxide. Glutathione reduces one of the oxygen atoms in the H2O2 all the way to water. Oxygen is taking electrons for the third time. The glutathione becomes oxidized in the process. When glutathione is recycled back to reduced glutathione – ready to reduce another H2O2 – a molecule of NADPH is converted to NADP+ (oxidized). The enzyme NNT then uses the oxidized NADP+ to convert an NADH to NAD+, supplying the necessary cofactor to continue running the citric acid cycle.
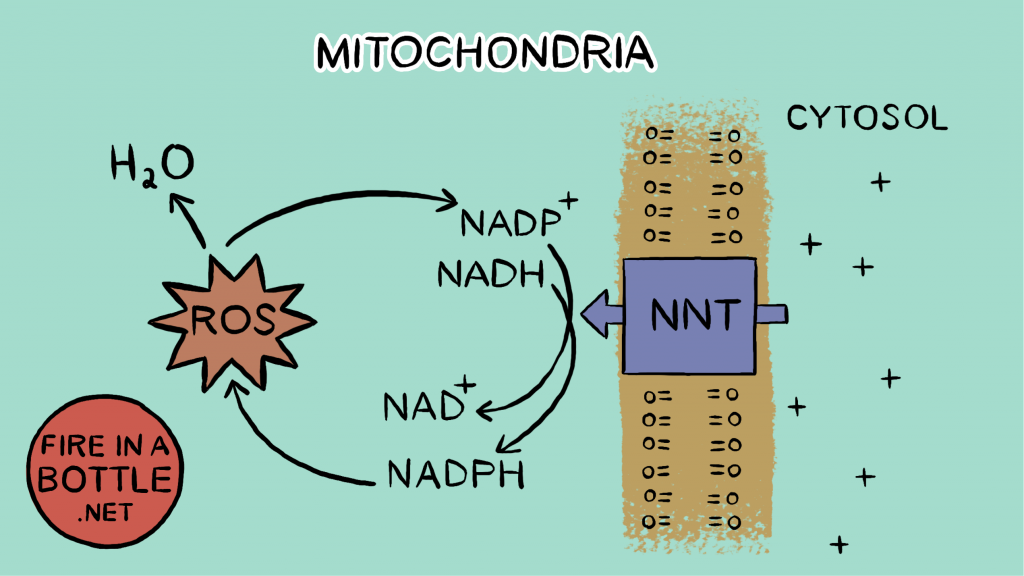
So the initial reduction of oxygen to superoxide sets off a chain of reactions, all of which reduce oxygen. The electron that formed superoxide, which “should have” been used to pump protons and increase reductive stress instead winds up oxidizing NADPH and reducing reductive stress.
The ultimate switcheroo.
Oxygen is Electronegative
The specialness of Oxygen is due to its high electronegativity, which is a measure of how strongly an element wants electrons. Oxygen (3.5 – upper right hand corner in red) has the second highest electronegativity of any element.
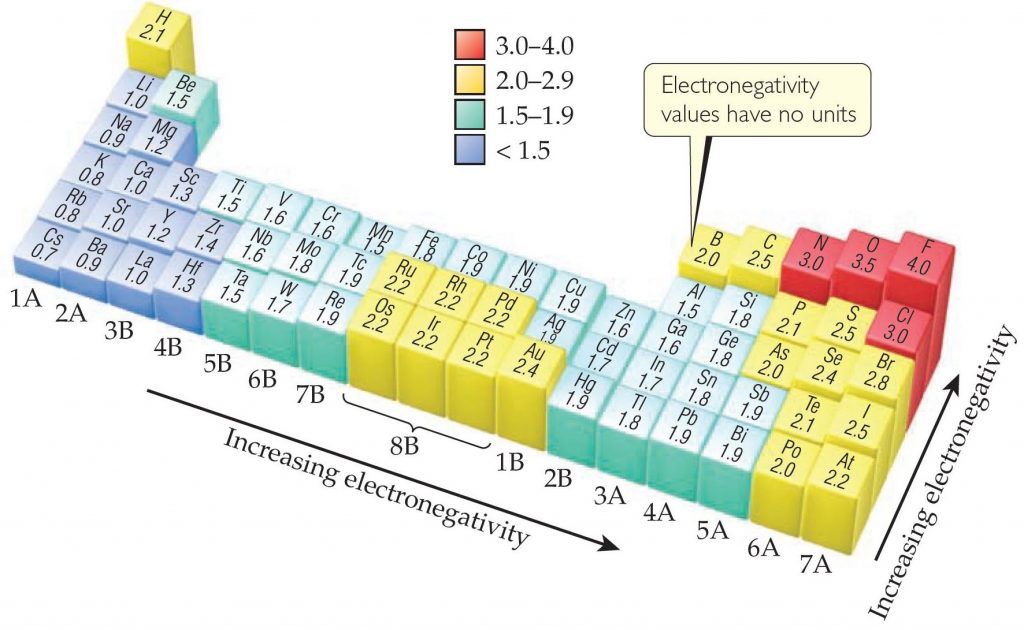
The only element more electronegative than Oxygen is fluorine, and fluorine is so reactive that a tiny poof of flourine gas will eat a hole through steel wool.
Oxygen is oddly stable given its high electronegativity and the fact that molecular oxygen (O2) is a triplet diradical.2 It seems that picking up that first extra electron to become superoxide is the key to it reaching its destiny.
Unsaturated fat causes reductive stress
The starch eating human will have a relatively high metabolic rate due to mitochondrial superoxide generation. The superoxide will regenerate NAD+ and keep things humming. The Tsimane farmer-foragers, who live on manioc and sorghum, are an example of this.3
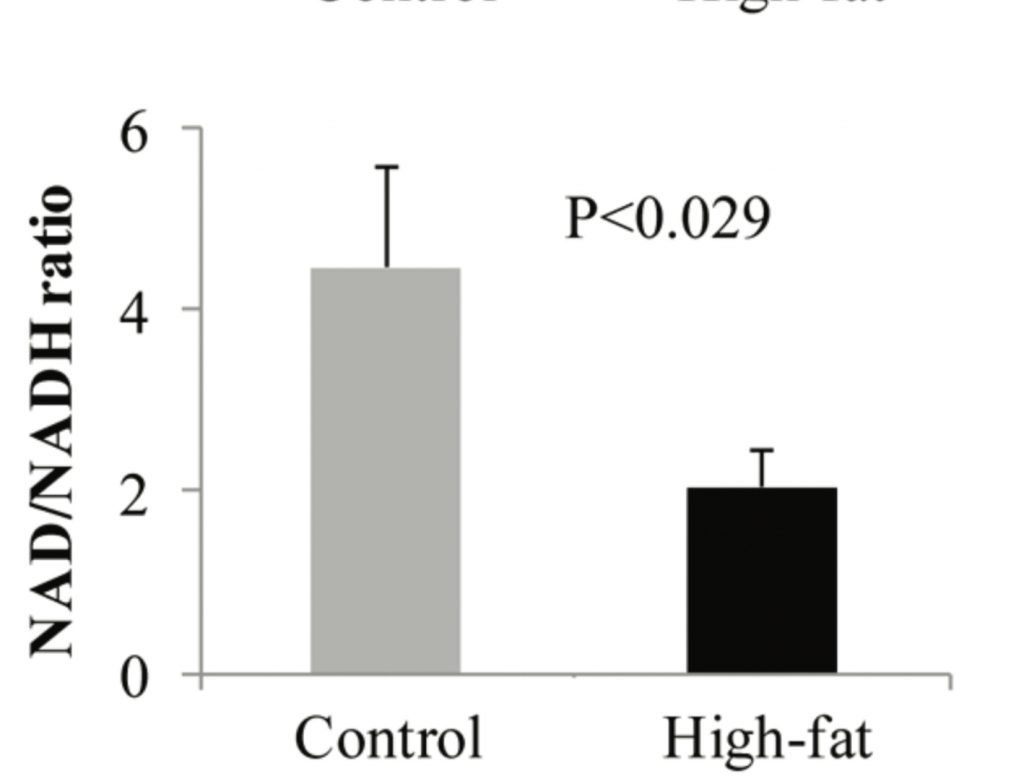
Unsaturated fats reduce mitochondrial superoxide production by reducing succinate dehydrogenase activity. In the lab we can cause obesity in mice by giving them a high fat diet composed mostly of high-PUFA, corn fed lard spiked with a little soybean oil. Look what happens to the NAD+/NADH ratio in these mice.4
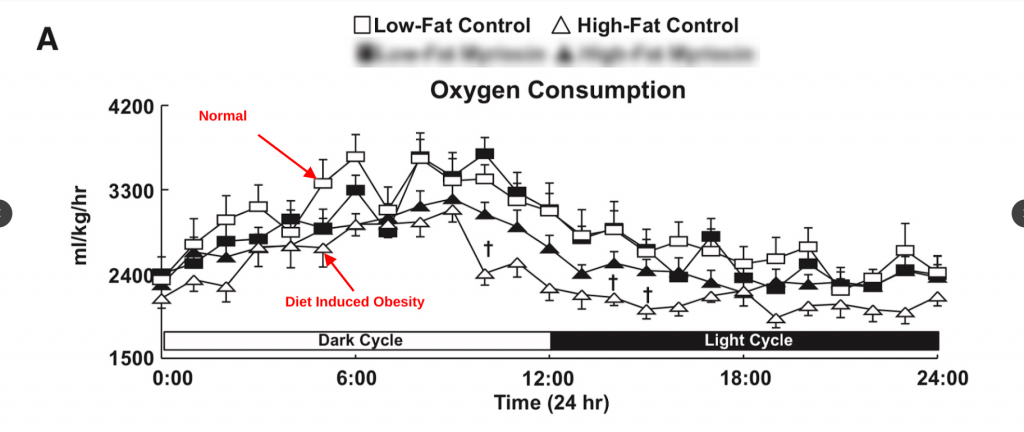
Mice fed unsaturated fat also become hypometabolic.5 (Ignore the solid black shapes.)
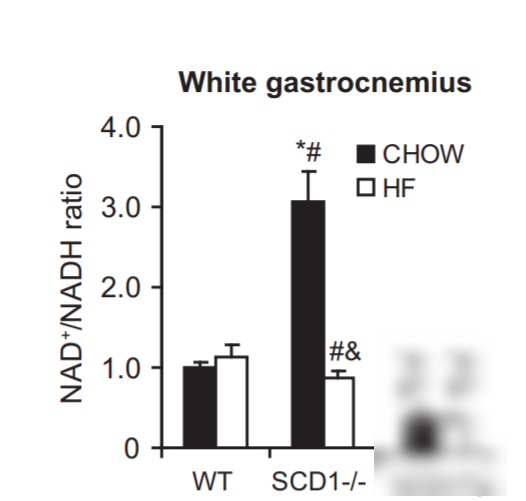
Conversely, in mice who lack the enzyme SCD1 and who cannot unsaturate their own fat, they have a massive spike in NAD+/NADH ratio that can be reversed by feeding unsaturated fat. SCD1 deficient mice have very saturated bodyfat, high NAD+ ratios and are hypermetabolic.6
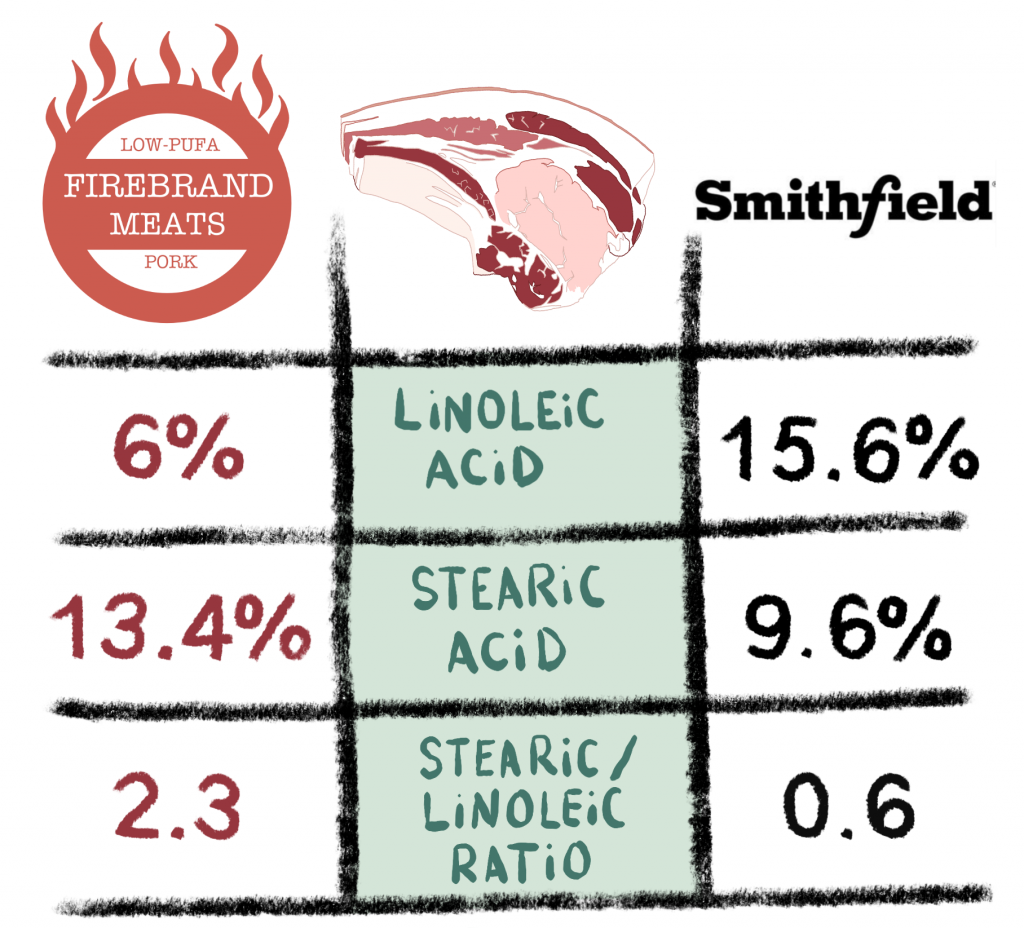
How unsaturated is most lard? I recently sent a sample of my Firebrand Meats low-PUFA Pork fat and a sample from Smithfield. The difference is quite astounding! It is currently shipping to the lower 48 states.
Conclusion
The unique properties of oxygen allow for a reductive stress pressure relief valve. When oxygen picks up an electron through Reverse Electron Transport, it will take two more on the way to becoming water. This behavior is very different than that of other redox couples. Regeneration of NAD+ is the key to a brisk metabolic rate. Unsaturated fat prevents Reverse Electron Transport, leading to low levels of NAD+ and reductive stress.
NEXT UP: Antioxidants cause reductive stress!
- 1.Azadmanesh J, Borgstahl G. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants. Published online January 30, 2018:25. doi:10.3390/antiox7020025
- 2.Borden WT, Hoffmann R, Stuyver T, Chen B. Dioxygen: What Makes This Triplet Diradical Kinetically Persistent? J Am Chem Soc. Published online June 14, 2017:9010-9018. doi:10.1021/jacs.7b04232
- 3.Gurven MD, Trumble BC, Stieglitz J, et al. High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. Am J Phys Anthropol. Published online July 4, 2016:414-425. doi:10.1002/ajpa.23040
- 4.Kim HJ, Kim JH, Noh S, et al. Metabolomic Analysis of Livers and Serum from High-Fat Diet Induced Obese Mice. J Proteome Res. Published online November 24, 2010:722-731. doi:10.1021/pr100892r
- 5.Ussher JR, Koves TR, Cadete VJJ, et al. Inhibition of De Novo Ceramide Synthesis Reverses Diet-Induced Insulin Resistance and Enhances Whole-Body Oxygen Consumption. Diabetes. Published online June 3, 2010:2453-2464. doi:10.2337/db09-1293
- 6.Dziewulska A, Dobosz AM, Dobrzyn A, et al. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J Cell Physiol. Published online June 26, 2019:1129-1140. doi:10.1002/jcp.29026

That is amazing for your pork fat. I keep fat from your pig meat, and throw it away from others.
Hi Brad.
So what can you suggest to us, who are post obese , who’s fat is quite unsaturated ? I tried to eat tcd , starch with butter . But I gain weight . Should I try hi fat feast/omad? What to try for fat loss and have energy ?
Your blog is great , thank you .
Yes, this is the question, isn’t it? The million dollar question. Here are some random thoughts, in no particular order…
1) Supplement with succinate/stearic acid/sterculia oil to mimick that you have more saturated body fat.
2) Try a low fat diet. I’m actually just starting a trial of low fat (lean meat and potatoes, mostly. Lots of gelatin.) supplemented with all three of the above plus lipoic acid and vit D and K2. The idea here is a low fat diet that mimics a high fat diet through the magic of succinate. My fear with this approach is that my body will simply make the starch into MUFA, but I’m using some sterculia oil so we’ll see.
3) Coconut oil is interesting and I’m mentioning it here first although my personal results with it have been underwhelming to date. Most sources of fat that aren’t high in PUFA – beef fat for instance – are also high in MUFA, which if you are post-obese you already have too much of. Coconut oil has very little MUFA AND almost none of the saturated fat is the right length to be converted to oleic acid by SCD1. Using coconut oil might potentially limit the fuel for SCD1 – I’ve seen studies in pigs where the 12 carbon and 14 carbon fats become 10% of body fat, which would limit to some extent, the ability of SCD1 to crank out MUFA.
4) Pu-ehr tea.
Just some thoughts, good luck and thank you!
Brad
Hi Brad,
Does your low fat experiment go against “Reduce SIRT1 and AMPK activity with low-fat calorie restriction, Increase them with high-fat feasting!” or is your situation different because calories aren’t restricted? Sufficient calories are of course twice as hard to get on low fat than high fat, and presumably getting sufficient calories will mean your carbs are through the roof… but maybe that’s not an issue? I guess that’s why it’s called an experiment. 🙂
I suspect the calorie load counts. Brad’s other post on starch – https://fireinabottle.net/ampk-is-activated-by-high-cellular-energy-after-a-starchy-meal/ – explains how the anti-torpor pathways (lol my shorthand for what we’re doing here) kick in if ROS is already high and you start flooding cells with glucose.
This also suggests a mechanism for something I heard on a Dr. John McDougall lecture- that eating too much starch in a meal isn’t a big problem because your body “burns off the excess”. McDougall makes a lot of bold statements he’s supposedly sure about but I see a mechanism here for how this is possible. Requires high ROS though- Stearic acid does it for me, but succinate might as well as ALA too.
Yeah, I think the “excess starch is burned off” is true of people with healthy metabolisms but in people who are torpid/post-obese it tends to trigger De Novo Lipogenesis instead. Obviously I’m hoping Succinate and sterculia oil can prevent the DNL.
Yes, experiment indeed! I’m hoping that ROS from the succinate will trick my mitochondria into thinking that I’m high fat feasting and I’m also supplementing with stearic acid.
Brad
The first time I became interested with NAD is the the time I watched the video of Dr. Sinclair last 3 years ago. According to him while human aged, NAD depletion happen depletion occur due to somewhat wrong understanding of mixing the recipe (in the cells view) Since I watched you in the podcast of Dr. Paul Saladino last year…I follow your blogs religiously. I am kind of stitching all the thing I learned these last 4 years I am trying to improve my health condition, I have tried low carb, keto, and carnivore, through these way of eating I remisioned my diabetes complications. What I want now is to completely reverse my diabetes of course through Him.
I would like to become an efficient starch burner. In my mind that represents true metabolic flexibility.
So says Ray Peat…..or at least that is my attempt to understand
TBH- now some 6 weeks into a McDougall-inspired high starch diet- I suspect it’s easier than you think it is, catch is you just have to commit to 70+% starch in your diet. Anecdotally stearic acid helps a lot (think- saute veggies in stearic enriched butter and top your rice with it). The low fat content and elevated metabolism from the starch seems to be “ratcheting down” my figure (weight has gone down a tad too) especially when I do mix hiking/exercise in my routine now.
Also- Biggest problems with going 75% starch for me:
1. The volume of food you must eat is insane. 3-3.5 cups of rice or equiv each meal (3 meals/day) with your veggies and very modest amount of meat (if any).
2. Occasional indigestion from the high volume of starch. Gassyness too. I bet if I dialed down the volume and did more than 3 meals/day this would be easier. I don’t think that’s a good idea because I believe insulin spikes are best kept minimal, and the hypercaloric glucose state stimulates AMPK/NNT/SIRT3/etc – the bigger the bolus the more of those calories get “wasted” IMO which is what we want. Fewer insulin spikes with a large glucose-area-under-the-curve overall.
Can you please make the succinade without sweeteners, natural flavors and stuff? A flavourless option. A lot of hypersensitive people out there such as myself who struggle to find good products without tons of fillers, flavorings and additives and stuff. Love your work,
I have a plan to offer the all-natural disodium succinate, which is created in a fermentation. The other disodium succinate products on the market are made from petroleum.
Brad
Molecules don’t have memories, they have configurations. The only detectable differences between ‘natural’ (extracted from plant/fungus/animal tissue) and ‘artificial’ (created in bulk processes or directed processes from precursors) are in the contaminants. (L-alpha-lipoic acid, or the original benzene test for examples)
Some of us would be willing to buy personal-size lots of the ‘artificial’ bulk product from you, or if you could point us at some relatively inexpensive suppliers willing to do so, it would be appreciated. (It shouldn’t prevent you from selling the ‘natural’ version for those who don’t believe my first sentence as well once you can source that.)
I tent to agree with you, but still I suspect most people don’t want petroleum based food. I am working on sourcing this.
Brad
Since my wife decided to bake the French Way I am doomed.
Croissants, butter pie crust cheese/mushroom quiche, lemon bars….and more.
Damned pandemic
Hapless
I bought the succinade, sterculia oil and plan to order some Rala soon. I also ordered fire starter from Saladino since you were out of steric acid for so long. I haven’t really taken everything simultaneously yet. Do you think all are necessary? Should I just avoid pufas or pufas and carbs?? I have been avoiding pufas since March. I am still very insulin resistant!! Ugh!!
If you are producing a lot of SCD1, avoiding PUFA alone probably won’t give you back a lean metabolism. I am currently just beginning a trial where I am using all three. We’ll see what happens!
Brad
Do you still have DI’s off the chart?
I was poking around wikipedia about ethanol metabolism. Interestingly, (again according to wikipedia) low NAD+ levels causes the acetaldehyde buildup that makes you drunk. I used to have a rock-solid alcohol tolerance, which was seriously reduced while I was doing keto, especially towards the end. On TCD-style eating it has been significantly restored. I’m curious if you have any anecdotes on this topic?
Another thought I have is on potential hormetic effects. On paper ethanol has a very low FADH2:NADH ratio, but we also know that habitual drinkers have better alcohol tolerance. So, could it be that alcohol hormetically (through some unidentified mechanism) pulls you away from reductive stress?
https://en.wikipedia.org/wiki/Microsomal_ethanol_oxidizing_system
Start drinking….hard
The acetaldehyde does not make you drunk, but is, together with the dehydration effect and excessive lactate production, mainly responsible for the hangover feeling afterwards. Look up ‘RU-21’, a Russian hangover prevention cocktail. Main ingredient : succunic acid. It does not prevent you from getting drunk, but the hangover is *much* easier if you take a glass of desolved succunic acid in soda water with each pint of alcohol : takes care of both the dehydration and prevents the buildup of acetyldehyde and lactate.
This is a fascinating anecdote. Thank you.
Brad
If you like that anecdote, here is another. Athletes in general but bodybuilders specifically also use succinic acid to supress the lactic acidosis after an intense workout. They then can go further and faster and reduce recovery time. As with all of these anecdotes, I tried them, and, they work.
Succinic acid can also mitigate some of the negative side effect of berberin/metformin.
Ethanol is a tricky one. This recent study showed that mice given a rather immoderate 8% ethanol in their drinking water consumed literally THREE TIMES the calories of teetotaling mice and weighed less. Needless to say I find this fact amusing. Eat less, move more? Why this happens, I’m still not exactly sure. It COULD be that ethanol is primarily metabolized in the liver and the liver is offloading to NADH via lactate and the other tissues are generating ROS via the glycerophosphate shuttle, but that is quite speculative.
Brad
Hi Brad,
Amazing work as usual buddy!!! I may have missed this somewhere in your articles but I was wondering how Protein works in all of this??? It makes a lot of sense to me how the above works with different energy sources but I wondered how protein mixes in with it all???
Progress Report:
Since taking 2400mg of R-Alpha Lipoic Acid + 2 squeezes of sterculia oil + 4 scoops of succinate a day my results are going amazingly!! I partner all of this with a very high protein diet of about 300g – 325g a day and I am certain I am slapping on muscle quickly while saturating my fat / burning it away!! Will be taking out Astaxanthin from my supplement stack after seeing how anti oxidants work against you.
Any other tips would be most welcome and thank you again for all of your hard work!!
Generally protein is left out in these discussions because your body tries to avoid catabolizing proteins, so the bulk of calories is coming from fats or carbs. In your case it’s probably more relevant.
I have to ask why on earth you are macrodosing everything you mentioned there, lol. Most people taking those are doing like 300-600 mg ALA and 1-2 scoops succinate. 300 g of protein a day??? I hope you understand how insanely high that is. And all at once??
Thanks for the report! I am starting a similar experiment to what you are doing. I haven’t really though too much about protein, other than this. High circulating levels of branched chain amino acids seemed to be used very preferentially for lipogenesis. Interestingly, it seems one reason that BCAAs build up is due to lack of lipoic acid, which is an enzyme cofactor in the enzyme that catabolizes the BCAAs. Having said that, it’s not clear that supplemental ALA winds up in enzymes. Anyway, can huge amounts of protein increase circulating BCAAs? I don’t know, but if it’s working for you I wouldn’t worry about it!
Brad
The 300g of protein is spread out between 3 big meals a day and is part of my muscle building program (currently train 6 days a week first thing in the morning). I weigh about 19 stone so the 300g is not all that shocking and I am a big fan of Kevin Stock & Ted Naiman’s protein focused work (might be worth a read if you are not sure who they are).
Are 2 squeezes of the sterculia all that much??? In fairness this is the one I want to drop down slightly as fat loss accelerates.
Brad mentioned with the succinate that the effects seemed to coincide with a percentage of calories for the day (2% I think he said for effects to kick in on weight loss during those studies based on 10g of succinate for 2000 cals a day) I have 3500 – 4000 everyday so this seemed logical to me. That being said this is all spread out on a 2 in the morning, 1 before lunch and 1 before dinner system.
Thanks for your thoughts on the protein side of things, that makes sense that I wouldnt really use it for energy as I am not in an energy deficit but still wondered how it compared to Saturated Fat or Succinate etc.
Brad, how would you say supplementation with nicotinamide ribose plays into this as it is a NAD+ booster. I sometimes take a small amount fasted in the morning for energy (100mg – any more interferes with sleep). There has been discussion on /r/saturatedfat/ as to whether or not NR is helping or hindering what we are trying to achieve on TCD.
The stearic arrived from you, wow – talk about energy and satiety. Also on sterculia, 600-1200 ALA (makes me tired at 1200), berb (can only manage 300-600 daily), and Succi-nade off and on. Started with the stearic just a few days ago but will post progress on Reddit after a month. Thanks again for all the great info here.
I don’t have any problem with supplementing NR. It’s mostly just a math argument. As to NAD+/NADH ratio, it seems better to me to take some NADH out of the denominator and add the NAD+ back to the numerator rather then using NR to soleely add to the numerator.
Does this mean, the kcal are lost as heat in this scenario (of high ROS production) then?
Yes, see my article ROS as a thermogenic cycle.
Brad, thank you for your excellent blog, that I as lay person cannot fully understand. But what you think about that inhaled nebulised or IV hydrogen peroxide can elevate NAD+? There is long history of H2O2 therapy. Your reasoning leads to it.
Yeah, I am aware of H2O2 as a therapy. I haven’t thought much about it, but it certainly would seem to work the same way. H2O2 would presumably make its way into most tissues since it can diffuse through membranes.
Hey Brad, thanks for the great work as usual. Some of us over in /r/saturatedfat/ were discussing the use of NR (nicotinamide riboside) to boost NAD+. I take a small amount (100 – 150mg) fasted in the morning but some think it may short circuit what we are trying to achieve on TCD. Any thoughts on this? A lot of people are into NR that I want to turn on to TCD but I need a better understanding of how NR impacts the electron transport chain and superoxide production when practicing TCD.
Currently on Sterculia oil, succi-nade, berberine, ALA, and now the stearic that you just sent out. The stearic is amazing – satiating and I’ve already seen some weight loss in just the past 5 days.
I don’t have any problem with supplementing NR. It’s mostly just a math argument. As to NAD+/NADH ratio, it seems better to me to take some NADH out of the denominator and add the NAD+ back to the numerator rather then using NR to soleely add to the numerator.
Random passage from one of Ray Peat’s pages, https://raypeat.com/articles/articles/sugar-issues.shtml – thought this was interesting (quoting a few broken segments here):
—
Besides being one of the forms of sugar involved in ordinary energy production, interchangeable with glucose, fructose has some special functions, that aren’t as well performed by glucose.
…
It has been suggested (Jauniaux, et al., 2005) that the predominance of fructose rather than glucose in the embryo’s environment helps to maintain ATP and the oxidative state (cellular redox potential) during development in the low-oxygen environment.
…
When oxygen isn’t constantly removing electrons from cells (being chemically reduced by them) those electrons will react elsewhere, creating free radicals (including activated oxygen) and reduced iron, that will create inappropriate chemical reactions (Niknahad, et al., 1995; MacAllister, et al., 2011).
Stresses and poisons of many different types, interfering with the normal flow of electrons to oxygen, produce large amounts of free radicals, which can spread structural and chemical damage, involving all systems of the cell. Ethyl alcohol is a common potentially toxic substance that can have this effect, causing oxidative damage by allowing an excess of electrons to accumulate in the cell, shifting the cells’ balance away from the stable oxidized state.
Fructose has been known for many years to accelerate the oxidation of ethanol (by about 80%). Oxygen consumption in the presence of ethanol is increased by fructose more than by glucose (Thieden and Lundquist, 1967). Besides removing the alcohol from the body more quickly, it prevents the oxidative damage, by maintaining or restoring the cell’s redox balance, the relatively oxidized state of the NADH/NAD+, lactate/pyruvate, and GSH/GSSH systems. Although glucose has this stabilizing, pro-oxidative function in many situations, this is a general feature of fructose, sometimes allowing it to have the opposite effect of glucose on the cell’s redox state. It seems to be largely this generalized shift of the cell’s redox state towards oxidation that is behind the ability of a small amount of fructose to catalyze the more rapid oxidation of a large amount of glucose.
Besides protecting against the reductive stresses, fructose can also protect against the oxidative stress of increased hydrogen peroxide (Spasojevic, et al., 2009). Its metabolite, fructose 1,6-bisphosphate, is even more effective as an antioxidant.
Keeping the metabolic rate high has many benefits, including the rapid renewal of cells and their components, such as cholesterol and other lipids, and proteins, which are always susceptible to damage from oxidants, but the high metabolic rate also tends to keep the redox system in the proper balance, reducing the rate of oxidative damage.
—
Interesting passage. Perhaps this is why Campari is so sweet…