Vitamin D is the sunshine vitamin. When sunshine hits your skin, your body uses the UV rays to convert cholesterol into vitamin D. A theme of this blog has been that obese humans have a torpid metabolism evolutionarily linked to that of hibernating animals in winter. As autumn approaches, consumption of foods high in linoleic acid PUFA, such as acorns, trigger the torpid metabolism by activating a transcription factor (turns on other genes) called PPARy. After winter, the metabolism of a hibernating ground squirrel returns to normal.
If I were to design a system like this, I would make the sunshine vitamin compete directly with PPARy. That way, in late spring and early summer, when the days were long and the sun was high in the sky, the ground squirrel would be lean and energetic, free to raise its young with maximal energy. Then as fall approached, the sunshine vitamin would decline just as linoleic content of the diet increased due to a fresh crop of acorns.
In fact, The vitamin D receptor is a nuclear receptor superfamily member who directly counteracts the effects of PPARy both by competing for their shared binding partner RXR and suppressing PPARy gene expression.
In this series, I have been discussing the crucial role of activating AMPK to counteract the lipogenic (fat making) effects of PPARy and the AhR. Vitamin D activates AMPK by competing with PPARy.

Human Relevance
Low vitamin D levels have long been associated with human obesity. A recent review paper1 concluded that vitamin D deficiency is associated with obesity irrespective of age, latitude, cut-offs to define vitamin D deficiency and the Human Development Index of the study location.
Vitamin D activates AMPK
Certain strains of mice can be routinely fattened on a high fat, high sugar diet. The mice that grow fat on this diet have lower levels of phosphorylated (activated) AMPK (pAMPK).
This study2 showed that vitamin D could reverse the drop in levels of pAMPK. pAMPK levels dropped significantly in mice given a high fat, high sugar diet. In mice fed a vitamin D deficient high fat, high-sugar diet, pAMPK levels go even lower. If you supplement the diet with lots of vitamin D, pAMPK levels are returned to control levels!
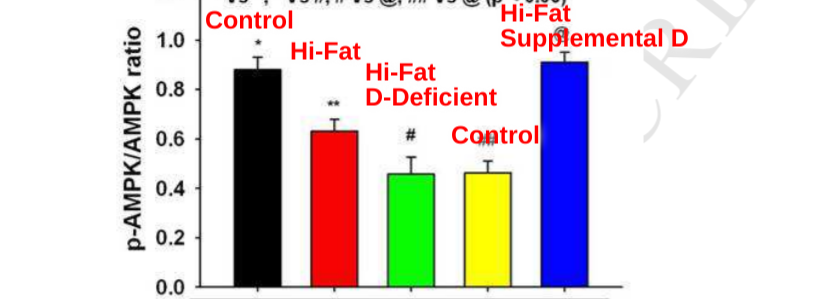
Vitamin D can help to prevent this. How?
Vitamin D Receptor competes with PPARy for its binding partner
Readers of this blog will have heard of the nuclear receptor superfamily. We’ve already seen family members PPARy, PPARa, the AhR and the FXR. Being a family, the nuclear receptors always do their job as a couple. They go into the nucleus, find a partner and turn other genes on or off. Like with every good family drama, the members compete with each other: for partners and for their ability to express themselves.
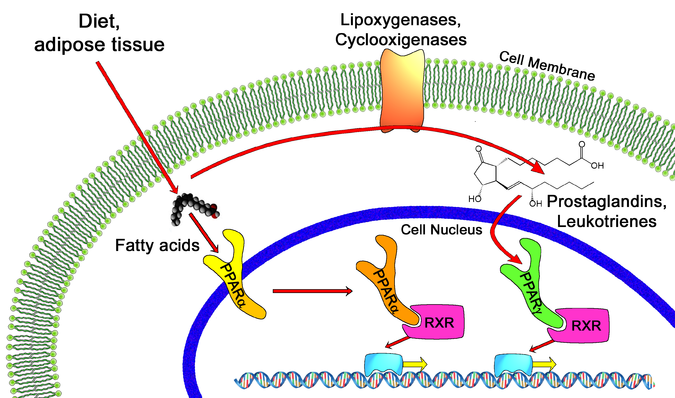
PPARy – the master regulator of lipogenesis (fat making) – and the vitamin D receptor (VDR) compete with each other for RXR, their binding partner. This study3 showed that vitamin D partially suppresses the ability of PPARy to turn on it’s target genes. If you overexpress the VDR in the presence of vitamin D, PPARy gene activation is blocked. If you then overexpress RXR – providing partners for everyone – the VDR can no longer block the activation of PPARy target genes.
Taken together, this is pretty good evidence that the vitamin D receptor and PPARy are directly competing for RXR and have the ability to outcompete one another. Vitamin D keeps SCD1 levels low and therefore pAMPK levels high by competing with PPARy for RXR.
Of course this is a very dynamic process and it’s all dependent on the nuclear receptors binding to their ligand (turns them on) – vitamin D in the case of the VDR, oxidized PUFA in the case of PPARy and retinoic acid (a vitamin A metabolite) in the case of RXR. So the amounts of VDR, PPARy and RXR can change but so do the amounts of their ligands.
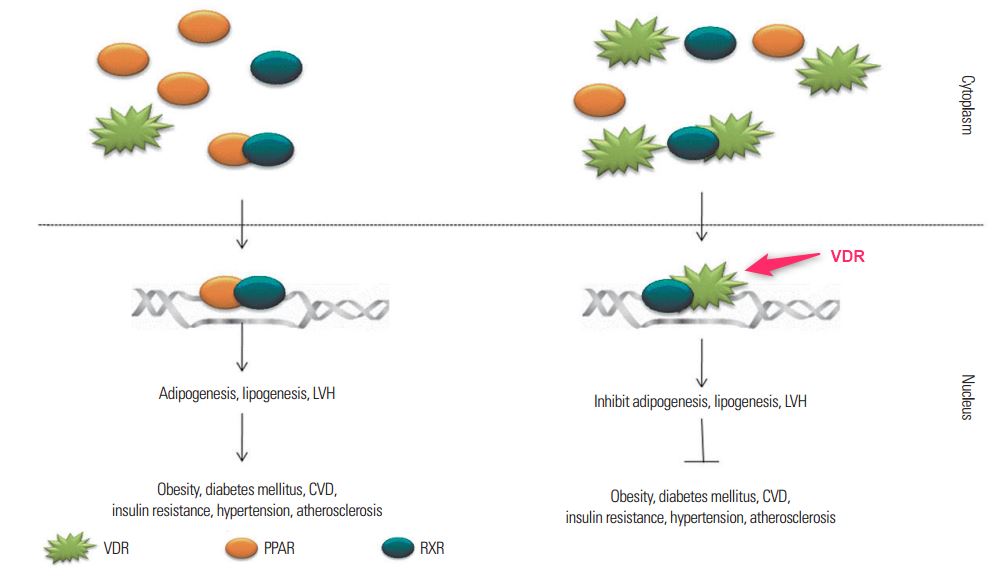
Vitamin D turns off PPARy and SCD1, and lowers fat storage
When the VDR is activated by vitamin D, it reduces the expression of PPARy. This means there is less PPARy around to compete for partners with. Vitamin D competes with PPARy on multiple levels. This leads ultimately to lower levels of SCD1. Lower SCD1 means higher levels of pAMPK.
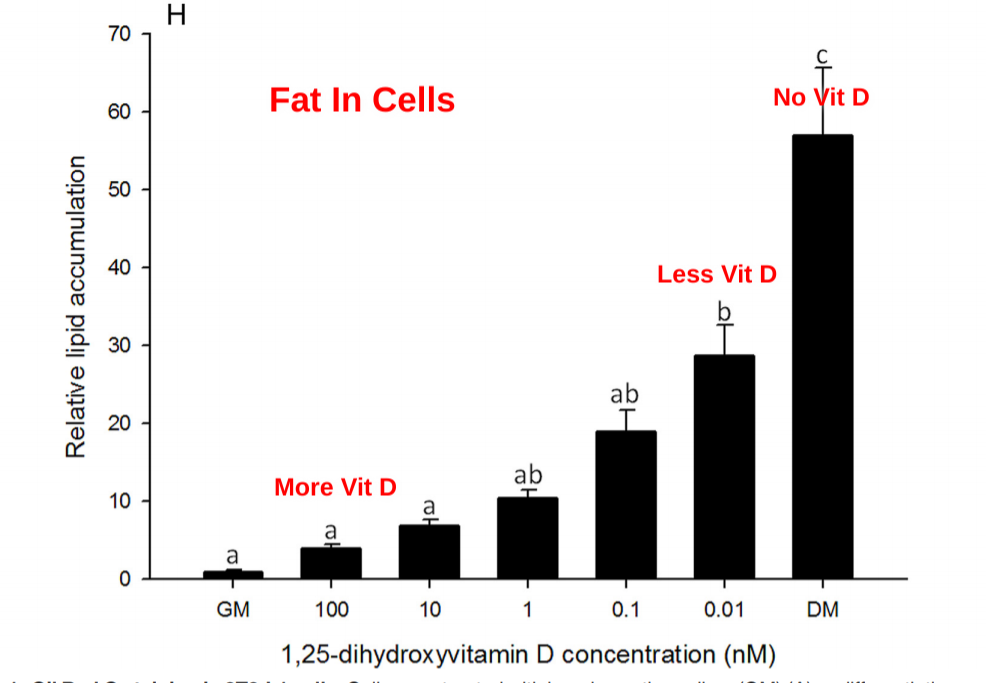
This paper4 demonstrates this nicely in tissue culture of human fat stem cells that are differentiating (growing up into mature fat cells). Vitamin D reduced PPARy and SCD1 – a master lipogenic gene – expression in the fat cells in a dose dependent manner.
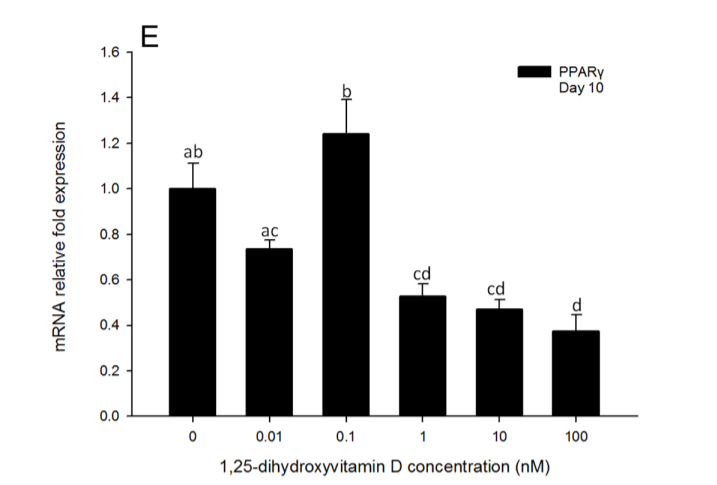
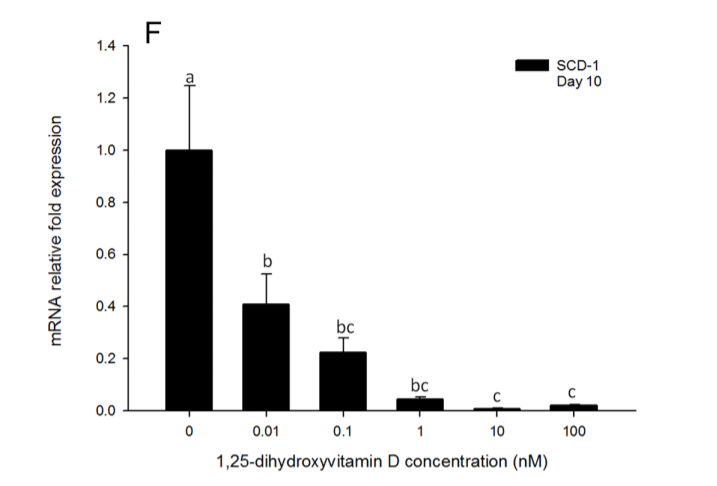
Vitamin D prevented fat accumulation in the fat cells in a dose dependent manner. For some reason the authors flipped the direction of the horizontal axis on this graph. Vitamin D levels are HIGHER on the left. I added the labels in red to try to help with the confusion.
What should I do with this knowledge?
If you don’t want a torpid metabolism, you have to stack the deck so that VDR can outcompete PPARy. The first step is to avoid consuming PUFA. Less PUFA around means less oxidized PUFA. That lowers the amount of the ligand of PPARy.
The second step is to get vitamin D! This means getting some serious sun exposure when you have the opportunity in the summer months and you should consider a high quality vitamin D supplement in the winter.
Get your desaturase index (DI) tested. The OmegaQuant complete test will give you an idea of your SCD1 levels based on the ratio of stearic acid to oleic acid. Just divide stearic by oleic. This is called the DI18. If you use the coupon code “fire” you will get a 10% discount on any OmegaQuant tests. If you want to do multiple tests to see how your DI changes you can get the two pack bundle and save even more. The DI18 is an indirect indicator of PPARy signalling. You can also calculate your DI1600 by dividing palmitoleic acid by palmitic acid and multiplying by 100. You can compare your DI18 and DI1600 to different cultures and to myself here.
If you have reason to suspect that you have overactive PPARy signalling, such as a high desaturase index, low body temperature, obesity, etc, you could consider limiting total vitamin A consumption, which would limit the activity of RXR. I’m wary of this approach because vitamin A has other benefits, but if your PPARy signalling really is out of control, perhaps it’s worth considering for a short period.
Lastly, consider any environmental effects that could be activating your AhR. The AhR oxidizes PUFA, providing ligands for PPARy. Oxidized LDL activates the AhR. You can get a test for OxLDL over at ownyourlabs.com. Compounds such as dioxins and PCBs also activate the AhR. So if you live downstream of a coal plant or incinerator or eat a lot of freshwater fish in polluted water, consider doing something about that.
Conclusions
Hibernating animals become torpid as winter approaches – as vitamin D levels fall. They become euthemic (normal body temperature) in the spring as vitamin D levels rise. Vitamin D competes directly with PPARy. Obese humans are low in vitamin D.
Vitamin D increases activated AMPK in hi-fat diet fed mice and reduces PPARy and SCD1 levels in differentiating fat cells, causing them to store less fat.
- 1.Pereira-Santos M, Costa PRF, Assis AMO, Santos CAST, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. Published online February 17, 2015:341-349. doi:10.1111/obr.12239
- 2.Manna P, Achari AE, Jain SK. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Archives of Biochemistry and Biophysics. Published online February 2017:22-34. doi:10.1016/j.abb.2017.01.002
- 3.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3inhibition of adipogenesis in 3T3-L1 cells. American Journal of Physiology-Endocrinology and Metabolism. Published online May 2006:E916-E924. doi:10.1152/ajpendo.00410.2005
- 4.Ji S, Doumit ME, Hill RA. Regulation of Adipogenesis and Key Adipogenic Gene Expression by 1, 25-Dihydroxyvitamin D in 3T3-L1 Cells. Seoane LM, ed. PLoS ONE. Published online June 1, 2015:e0126142. doi:10.1371/journal.pone.0126142

So neat to see the sunshine effect in the flesh!
I read it as VitD helps prevent the blocking of AMPK…
Now on to what upregulates RXR..
Been taking vitamin D a long time and I’m still fat.
What’s the effect size here?
Yeah, I suspect that to regain a normal lean metabolism, we will need to use a handful of approaches. Clearly in most people simply supplementing vitamin D doesn’t cause massive weight loss. OTOH, if you have low D status it could be causing your weight loss to stall.
You could be deficient in copper or other minerals. Have you had your levels tested to see if the supplementation is effective? I’ve really been liking the info in “the root cause protocol” for mineral/vit info…
This makes an interesting connection to Grant Genereux’s Vitamin A toxicity hypothesis. Since coming across his material, it has seemed to me that the idea that “vitamin A in all forms and all doses is a toxin, period” takes things just a bit too far given how common vitamin A is in traditionally consumed and highly valued foods. If I’m interpreting this correctly, it would seem that vitamin A could indeed be problematic for someone consuming a high-PUFA diet and minimizing sun exposure, as many Americans do.
I’m eating some beef liver crisps right now… 1 serving is 100 calories and 3710mg of vit. A (410% DV).
I’m with you on his all/nothing idea. It goes against logic to think there are so many things vital to good health stored in the liver, but Vit A is not one of them? It might be a problem for people consuming high PUFA’s and low in D, but in a healthy person I can’t see where it would not be beneficial, much less detrimental. Especially when it comes from a whole food and not a synthesized form taken in isolation.
I’m gonna keep eating my liver crisps that’s for sure!
Yeah, eat your liver crisps. They’re also packed with B vitamins, choline, etc. I suspect at the end of the day vitamin A is overall beneficial. Probably the solution is more vitamin D and less oxidized PUFA rather than eliminating vitamin A.
strange…..as i’ve been relatively scrupulous about eliminating PUFA N6 my vitamin D status has improved from already decent levels. and my basal body temperatures are rising. I live in a weather Shangrilah with lots of sun and I get out in it. Winter does not exist like in New Yawk. Current phase finally gets the green light to supplement EPA/DHA and adding Sterculic Acid. And enjoying a smattering of croissants and some after dinner Apero.
What actually is a reasonable level of Vit D? Not sure anyone really knows.